
Garden and Plate
The Molecular Biology of Nutrition










With the possible exception of some fruit and vegetables, plants don't create their nutrients with us in mind. Sometimes they even create obstacles like poison or hard shells to keep us away. The foods we eat aren't always best for us in their natural form, so we sometimes need to optimize them for our use. That doesn't mean we should over process them until most of the nutrients are gone, but it does mean some preparation is often necessary. We may want to cook potatoes to break down cell walls, or enlist the assistance of yeast when we bake our bread. We can't even digest our broccoli without help from the bacteria in our gut.

A single plant may not supply all of our nutritional needs, so eating a combination of foods is required for a balanced diet. Plants and people share many nutritional components and molecular processes, so most foods require only moderate preparation. If we want to store them, however, we also need to know how to preserve them. This section looks at food as it makes it's way to our plate. We examine the nutritional content of food and how it's preparation and storage affect the nutrients within.


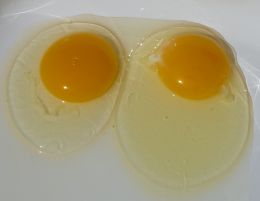
Hens don't have omletes in mind when they lay their eggs. They are putting together a sack lunch for their chicks. Once the egg is laid, a chick must go from a single-celled organism to a fully-formed chick, using only the nutrition that's included inside the egg. Just as a plant packs plenty of nutrition into a seed to support the seeds early growth, hens pack lots of nutrients into an egg. It's like a seed for growing meat. This makes the egg a powerhouse of nutrition for any organism that's made out of meat, including us.
|
The nutritional needs of chicks are in many ways similar to the nutritional needs of humans, so eggs are a great addition to the human diet. Both of us need to build bones and muscles, and we use many of the same proteins. Even when we don't, we build our species-specific proteins from the same amino acids. Feathers and hair are different in structure, but they are both made from protein. Then, of course, there's all of the vitamins and minerals. Since eggs are such an important part of many people's diets, we should see what the hen packed into this sack lunch of hers.
For starters, an egg provides all of the essential amino acids in the right proportions (the same as most meats), so eggs are a more complete source of protein than most plant foods. They are also high in calories, because building a chick from scratch takes lots of energy. Chicks (like all living things) are made mostly of water, so there's plenty of that. Going from a single-celled organism to a fully developed chick requires the building of a huge number of cell walls, so the infamous (but nutritionally essential) cholesterol is also present.
The edible part of the egg is divided into two nutritionally (and visually) distinct sections; the egg white and the yolk. Water and protein are found in large amounts in both the white and the yolk, while most of the fat, vitamins, and minerals are found in the yolk. Cholesterol is a fat, and hangs out exclusively in the yolk.
According to the USDA nutrient library, a 50 gram (g) large chicken egg contains approximately 38g water, 6g protein, 5g fat, .186g of cholesterol and .18g sugar. It also provides several vitamins and minerals, and 72 kcals of energy. The USDA also provides nutritional information for the egg yolk and white separately.
Egg shells are produced in several colors, and are a product of a hen's genetic heritage. The shell colors don't have an effect on an egg's flavor or nutritional content. The orange color of the yolk, however, is derived from plant pigments (called xanthophylls) that are in the chicken's feed. The orange color of the yolk can be made lighter or darker by changing the plants used in the hen's diet.
Glucose, fructose, sucrose, high fructose corn syrup, table sugar or simple carbohydrate. Take your pick. There are many different words for sugar. Do they all refer to the same thing, or are there significant nutritional differences? Lets compare some of them to find out.


There's been a great deal of controversy regarding the health effects of using High Fructose Corn Syrup (HFCS) in our foods as a replacement for table sugar (sucrose). HFCS has a reputation as an "artificially manufactured" replacement for the "natural" table sugar we harvest from sugar cane and sugar beets. Corn subsides have made HFCS less expensive to produce than sucrose, so it's been finding it's way into more and more of our foods. They both contain fructose, which is sweeter than glucose, so they compete for the affections of our sweet tooth. But are they really so different? Since we consume them in such large quantities, it's important to find out if one is better for you than the other.
|
Sucrose, which is best known as table sugar, is derived mostly from sugar beets or sugar cane. Sucrose is a disaccharide that's composed of glucose and fructose monosaccharides that are linked together by an ether bond. Only monosaccharides can be transferred from the intestine into the bloodstream, so an enzyme called sucrase breaks the ether bond, resulting in a 50/50 mixture of glucose and fructose.
High fructose corn syrup (HFCS) is derived from corn syrup. The carbohydrates in corn are almost entirely composed of starch, which is broken down into it's individual glucose monosaccharides as a first step in the creation of HFCS. The glucose isomerase enzyme is then used to convert 42% of the glucose into fructose. The relative levels of fructose and glucose can then be adjusted to whatever percentages are desired. Two concentrations are commonly used in our food supply. 42% HFCS is used in many baking applications, while a 55% fructose mixture is used as a sweetner in many foods, including soft drinks.
The only difference between sucrose and HFCS is the slightly different concentration of fructose (50% in sucrose vs 42% or 55% in HFCS), and the fact that sucrose must be broken down in the intestine into monosaccharides before it can be absorbed, while HFCS is already broken down. Sucrose is easily broken down by the sucrase enzyme in the intestine, so in both cases you end up with an almost identical mixture of glucose and fructose entering the bloodstream.
We've compared Sucrose to HFCS and found no significant nutritional differences. Both are composed of nearly identical amounts of fructose and glucose. But how does fructose compare to glucose? Are there important nutritional differences between these two monosaccharides?
Glucose and fructose are chemically identical (C6O6H12), but structurally different. This has important nutritional consequences. Glucose can be broken down (metabolyzed) for energy by every cell of the body in a process called glycolysis. Fructose is structurally different, so it can't take part in glycolysis. Instead, it goes through a similar process called fructolysis, that takes place in the liver. While the processes are similar, the fact that they take place in different locations makes a big difference.
|
Glucose is used by every cell in the body, so most of it isn't processed by the liver. Fructose is processed only in the liver. Since the rest of the body uses more energy than the liver, most glucose gets used up quickly for energy, while most fructose gets stored as glycogen or fat. Glycogen can be converted into glucose and released into the bloodstream later, but it must be stored first; the liver can't convert fructose directly into glucose. This isn't a problem if a diet is low in fructose, but if it's consumed in large amounts, or in highly concentrated forms like sucrose or HFCS, it has negative consequences. The fat accumulates, and is seldom used.
Fructose isn't regulated the way glucose is. When glucose enters the bloodstream, it causes the pancreas to release insulin. This causes the fat cells to release a hormone called leptin. Insulin helps glucose enter the cells of the body, while leptin tells the brain it has had enough to eat. Fructose doesn't trigger the release of insulin or leptin. While glucose satisfies your hunger, fructose does not. This encourages over-eating.
According to the USDA nutrient library, one teaspoon (4.2g) of granulated sugar (sucrose) has 16 kcals of energy in it's 4.2 grams of pure carbohydrate. It contains no water, no protein, no vitamins, no fiber, and no minerals. But it's also fat free, so it must be good for us, right? Everything in moderation.

Water may not technically qualify as food all by itself, but unless you like to sit down to a dinner of beef jerky and saltine crackers, it occupies a prominent place on your plate.
Water makes up a large percentage of most foods. Mole-cular biology (not to mention digestion) would be impossible without it. Water might be just two hydrogens stuck to an oxygen atom, but it's more complicated than it looks. In fact, it's possible to devote an entire page to it.
If you just can't stay away from all of those incredible edible eggs, but are worried about too much cholesterol, replace that slice of toast with a bowl of oatmeal.

The claim that oatmeal lowers cholesterol is more than just marketing hype. Oats contain water soluble fiber that combines with bile. When the fiber and bile combine, the bile is eliminated from the body instead of being re-absorbed by the large intestine at the end of digestion. How does this lower cholesterol?
Bile is 70% cholesterol. When the bile isn't recycled, it must be replaced. Cholesterol from the bloodstream is used by the liver to make new bile, which is then stored in the gallbladder. This lowers cholesterol levels in the blood.
Bile is used as an aid to digestion. Water and oil won't mix without help, so the gallbladder releases bile, which works as an emulsifier.
People aren't the only creatures on earth that like to eat. Every living thing, no matter how small, needs to eat in order to survive. As soon as the first organisms found the first biological molecules, the struggle for their possession began. That's why living things eat each other. It's a competition for organic molecules.
That's why you can't leave most food unprotected without it spoiling. The micro-organisms eat it, metabolyzing the biological molecules into waste. Food preservation is mostly about keeping other organisms from eating the food, but also about preventing chemical reactions like oxidation and freezer burn from damaging the chemical structure of our foods. There are several ways to prevent both from happening, at least for a while.

Life needs liquid water in which to perform it's biological reactions. When water freezes, all biological activity ceases, and the food is preserved. Water expands when it freezes, which sometimes causes the cell membranes in food to burst. This is why some foods lose their texture when they are thawed out.
Food left in the freezer for too long can experience freezer burn, which is caused by sublimation. This is a process where water can go from a solid to a gas without passing through a liquid state. It's dehydration that occurs in the freezer.

The other end of the temperature scale also provides an effective way to preserve food, if you combine it with isolation.
Heat is the motion of atoms and molecules. If the temperature in a food rises too high, biological processes in the cell can't continue due to all of the thermal energy, and the cell dies. While cooking will kill all of the life in a food, it's not enough for food preservation over time. New microorganisms will arrive on your food as it cools, and re-colonize it.
To preserve food, you need to isolate it first, then heat it. This is the principle behind canning. Tuna fish will spoil in less than a day if left exposed. Seal it in a can and then apply heat. The tuna will then last for years.
Salt is an essential nutrient, but can be fatal in large amounts. Salt draws water towards it, which can cause dehydration. This is why salty foods make us thirsty. Drinking ocean water, for example, can kill you because the salt concentration in it is so high that it causes dehydration. Our bodies use more water to flush out the excess salt than we get from drinking the ocean's water.

If we combine dehydration of food with the addition of salt, we can preserve some foods by making them inhospitable to micro-organisms. That's the principle behind foods like beef jerky. Germs can't reproduce on foods that contain lots of salt and little water.

Pickling takes advantage of low PH levels to preserve food. Biological processes within cells function best when PH levels are at or near 7.0. If you change the PH level too far in either direction, the microorganisms can't survive. Pickling preserves food by placing it in vinegar, which has a PH level near 2.4.
Sometimes food spoils by simple chemical reactions, without any microorganisms being involved at all. Freezer burn is one way this happens, and food going rancid is another.
Freezer burn happens when frozen food is exposed to air. Ice evaporates from food in a process called sublimation. It doesn't have to melt into water first. This dehydrates the food, which lowers it's quality. Removing air pockets from your frozen containers will slow down this process.
Rancidity takes place when oxygen breaks apart the unsaturated (double) bonds that exist between some carbon atoms in the fatty acid chains of lipid molecules. This separates small foul-tasting fragments (aldehydes and ketones) from the fatty acid chains.
To prevent this from taking place, minimize the exposure of those foods that are high in unsaturated fats (like olive oil) to oxygen.

How are two toxic chemicals transformed into a nutrient so essential that it gets it's very own spot on our tastebuds? Find out here.