
Garden and Plate
The Molecular Biology of Nutrition










The purpose of this page is to provide a clear, concise and accurate overview of the fundamentals in chemistry and molecular biology. It covers the essential concepts that are necessary for understanding nutrition as it's presented in the other pages on this site. If you wish to delve deeper into these subjects, I've made a list of some of the best resources that I came across while doing research for this site. They will take you as deep as you want to go.
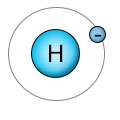
The blue center of the hydrogen atom (H) has one proton, and is surrounded by one electron. It has only one shell, which can hold up to two electrons, so it's looking for one additional electron.
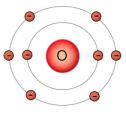
The red center of the oxygen (O) atom contains eight protons, and is surrounded by eight electrons. Two in it's inner shell, and six in it's outer shell. It's inner shell can hold only two electrons, so it's full. It's outer shell can hold up to eight electrons, so it's looking for two more electrons.
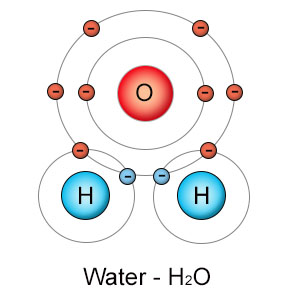
The oxygen atom needs two electrons, so it uses one from each of two hydrogen atoms. The hydrogen atoms each need one electron, so they each use one electron from the oxygen atom. Now all three atoms have full outer electron shells. This type of sharing is called a "covalent bond", and is the basis for the formation of all molecules. The molecule formed is water (H2O), which has properties that neither the hydrogen or oxygen atoms had on their own.
The physical world is made up of many different kinds of elements. A complete list of them can be found in the Periodic Table. A single instance of an element is an atom. Atoms have a center that's made up of one or more particles called protons. Atoms are defined by the number of protons they have. For example, hydrogen has one proton, while oxygen has eight. Protons have a "positive" charge (which you can think of as being similar to the attractive/repulsive effect of a magnet). There are usually neutrons in the center as well, but we can ignore them.
Protons are surrounded by "orbiting" electrons. Electrons are much smaller than protons, and have an equal, but opposite "negative" charge. The electrons are attracted to the protons (opposite charges attract), which is why they orbit the protons. The electrons are repelled by each other, which limits how many electrons can fit around the protons in a given area. The more protons in the center, the stronger the positive force. The stronger the force, the further out it reaches, allowing the atom to contain more electrons.
Because only a specific number of electrons can fit in close to the protons (because they repel each other), some of them have to sit farther out in layers called "shells". Each shell can hold only so many electrons. If a shell is partially full, it has empty spots. Atoms try to get rid of these empty spots by adding or releasing electrons to get to the point where their outer shell is full or empty. This is what causes chemistry.
Chemistry can be defined as the processes that take place when atoms share electrons. All nutritional processes are chemical reactions, so the study of nutrition at the most fundamental level is the study of how atoms share electrons. Since different atoms have different numbers of electrons, they have differently shaped empty spots in their outer shells. This makes them connect to different kinds of atoms in a variety of ways. You can think of them as Lego blocks, if you wish.
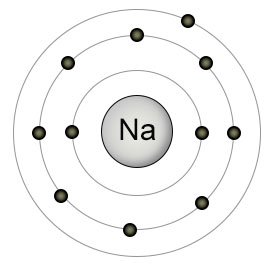
The center of the sodium atom (Na) has eleven protons, and is surrounded by eleven electrons. It has only one electron in it's outer shell, which it wants to get rid of.
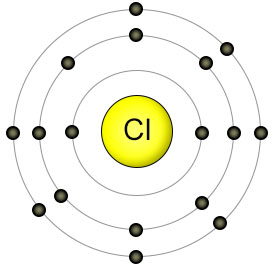
The center of the chloride (Cl) atom contains seventeen protons, and is surrounded by seventeen electrons. It has seven electrons in it's outer shell, and needs one more.
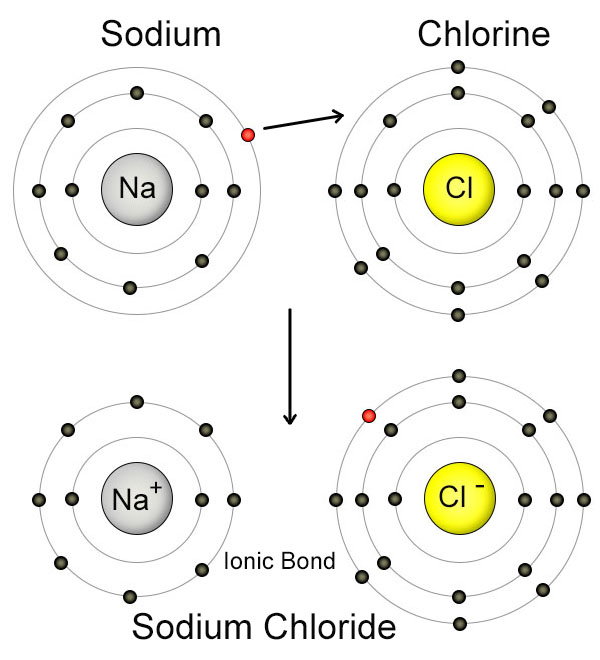
The sodium (Na) atom transfers one electron to the chlorine (Cl) atom, which gives both of them full outer shells. The sodium atom now has only ten electrons, but still has eleven protons. This upsets the balance of charges between the protons and electrons, which causes the sodium atom to have a net positive charge. When this happens, the atom is called a "positive ion". The chlorine atom now has more electrons than protons, so it's become a "negative ion". Because the sodium atom has a positive charge, and the chlorine atom has a negative charge, they are attracted to each other, and form an ionic bond. Ionic bonds form salts, not molecules, but they are still important in nutrition.
There are three different ways that atoms share electrons. The first is called "Metallic Bonding". Metals are atoms that have a weak hold on the electrons in their outer shell, so they give them up easily. When you have a high concentration of metal atoms (like in a copper wire), the outer shell electrons form a cloud, moving easily from one metal atom to another. This is why electricity flows so well through metal. I'm not aware of any chemical processes in molecular biology and nutrition that depend on metallic bonding.
The second way that atoms empty or fill their outer shells is by giving away or accepting electrons from other atoms. This is called "Ionic Bonding". When this happens, the atoms become "ions", which means that an atom has more (or less) electrons than protons. Since this is the case, the entire atom takes on a net positive or negative charge. A negatively charged ion has more electrons than protons, while a positively charged ion has less electrons than protons. Negative and positive ions are attracted to each other just like protons and electrons are, so they tend to form bonds. Table salt, which is created from a bond between a sodium ion and a chlorine ion is a good example. Ionic bonds are very important in nutrition.
The third way that atoms fill their outer shells by sharing electrons is called "Covalent bonding". Instead of giving up or receiving an electron, they interlock their outer shells by combining the electrons in partially filled outer shells in such a way as to give all of the atoms involved (There can be more than two) a filled outer shell. Covalent bonds are stronger than ionic bonds, and are the most important kind of bond in molecular biology and nutrition. A molecule is defined as two or more atoms, connected by a covalent bond. Since an atom can form covalent bonds with more than one other atom (depending on how many electrons or empty spots it has in it's partially filled outer shell), an unlimited number of atoms can be strung together into large molecules. For example, carbon has four electrons and four empty spots in it's outer shell, enabling each carbon atom to form covalent bonds with up to four other atoms (carbon or other kinds).
This video presentation from the Cassiopeia Project shows how atoms combine in different ways, through a variety of chemical bonds. Molecules form using covalent bonds, while ionic and hydrogen bonds create other kinds of non-molecular structures.
Now that you understand the basic structures of the atom and the different ways they share electrons in order to form molecules, you'll be able to follow the explanations in the other articles. Just as the twenty-six letters of the alphabet can be used to create an unlimited number of words, a small number of atoms can be combined in an unlimited number of ways to create all of the molecules that make life possible. The atoms used in these combinations are listed here. The details of how they are combined to create the molecules important in nutrition is the subject of all the other articles on this site.
The molecular processes described on this site are abstracted out of the complex environment of overlapping processes that arise out of the molecular soup within the cell. These processes are real, but they are not isolated from the many other chemical reactions that are going on simultaneously. The articles make them seem that way, because that's how we learn new things. We isolate to understand. In reality, the steps described in a process can be interferred with by re-routing a molecule from one process into another unrelated one, or by reversing a chemical change that just took place. This may sound inefficient (which it is), but it also provides a feedback loop that regulates how often a particular process takes place. This is how homeostasis (balance) is maintained in a cell.
The molecular processes are the result of millions of years of trial and error through a process of natural selection. No-one sat down and decided to design the process used for creating a carbohydrate, or any other molecule. This is stating the obvious, but it needs to be brought to our concious attention if we are to understand why things work the way they do. Most of the time we approach complex systems with the implicit assumption that it was designed. It's the mindset we automatically use when we try to understand something. You'll see that many of the processes are very convoluted. They are like rube goldberg machines, going through many tedious steps to make one simple change (for example, see the Calvin Cycle). Now you know why. This is how natural selection works.
Another thing to keep in mind is the language of Molecular Biology. It's full of large, unfamiliar words that can make the subject matter difficult to understand. But if you break it down to it's fundamentals, you see that it's made up of many simple parts. The words are difficult because they refer to unfamiliar things. Don't be intimidated by the language. When you start with the simple fundamentals and build from there, the complicated becomes understood. When we apply our understanding to the everyday world of gardening and food preparation, it becomes relevent. That's what we hope to accomplish on this site.
Molecules and salts are assembled from individual elements, using the covalent and ionic bonds described on this page. Details for each of the twenty-one elements that are considered essential for life can be found on the Elements page.
In addition to the three types of bonds (metallic, ionic and covalent) described on the left side of this page, there are also hydrogen bonds. Hydrogen bonds aren't a fourth way of sharing electrons between elements, but a relatively weak bond that forms between polar molecules. Molecules form these bonds with each other when the covalent bonds within the molecules don't share their electrons equally.
Not all atoms attract electrons with the same amount of force. Electrons are attracted to protons in the center of the atom. If one atom has more protons than another, it will have more "pull" than the other atom. In addition, the closer an electron can get to the protons, the stronger the attraction. So an atom with empty spots for electrons in it's second shell will pull electrons in closer than an atom that has empty spots in it's third shell. These two characteristics of an atom define it's "electronegativity" (It's attractive force on electrons shared with other atoms).
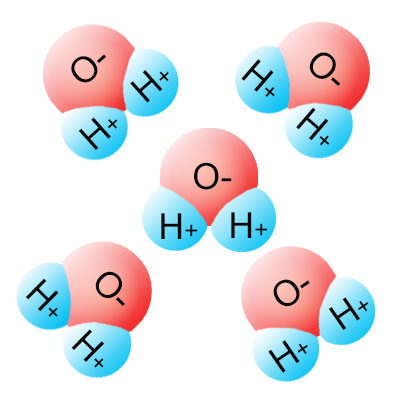
Hydrogen bonds form between molecules that don't share their electrons equally. The positive side of one molecule is attracted to the negative side of another.
Take water as an example. It's a molecule that's formed when one oxygen atom forms covalent bonds with two hydrogen atoms (see the images on the left side of this page). Hydrogen shares electrons in it's first shell, while oxygen shares electrons in it's second shell. All other things being equal, hydrogen should have stronger electronegativity (pull) than oxygen. All other things aren't equal though, because hydrogen has one proton, while oxygen has eight of them. The extra seven protons in oxygen more than make up for the shell differences, making oxygen more electronegative than hydrogen. This causes the shared electrons to be pulled closer to the oxygen side of the molecule than to the hydrogen side. Since electrons have a negative charge, the oxygen side of the water molecule is more negative than the hydrogen side, making it a polar molecule.
A final thing to consider is the molecular shape. For reasons too complicated to get into here, both hydrogen atoms position themselves on the same side of the oxygen atom, which increases the polarity of the water molecule. The end result is a water molecule that's highly polar. What does this mean? It means that every water molecule acts like a magnet, which is negatively charged on one side, and positively charged on the other. The positive side of the molecule will bond with the negative side of other polar molecules (not just water), and the negative side of the molecule will bond with the positive side of other molecules. This is why water tends to bead up into drops. The bonds are called hydrogen bonds because hydrogen is often (but not always) a component of polar molecules. Hydrogen bonds are critical in many biological processes, including how DNA and protein maintains it's shape.
The chemical processes that create, modify, and breakdown the major molecules like fats, proteins and carbohydrates, depend on several smaller molecules that are used over and over to make the chemical changes possible. You can think of these molecules as the grease that lubricates the machinery.
Organic molecules are organized into groups based on function and structural similarities. Each of the introductions below will take you to a page where a particular nutrient class is examined in detail.
This site is a work in progress. Some pages referenced below haven't been created yet.
Follow the adventures of a carbohydrate from it's creation in photosynthesis to the many different places it can go, and all of the forms it can take. From the energy in your cells to the wood in your furniture, carbo-hydrates really know how to get around.

Proteins are the machines of the biological world. They can move other molecules around, break them apart, and assemble them in new ways. Proteins only perform specific tasks, so there are thousands of different kinds. Form follows function, so each protein must fold into a specific shape before it can do it's job. Proteins practice yoga on a molecular scale.

Fats, oils, or lipids. Call them what you want, they do more than just collect at the waistline. They transport messages throughout the body and provide the structure for building cell walls. Most fats are created equal, but we aren't consuming them in equal amounts. We get too much of some, and not enough of others. Let's find out which ones are which, and what the differences are.
Vitamins are the tools of the molecular world. They enable enzymes to perform tasks that cannot be completed by a mere string of amino acids. We aren't able to create them ourselves, so must get them from our diet.
Minerals are the rocks of the biological world. They are individual elements, not molecules, so they are described in detail on the Elements page. They include potassium, phosphorus, iron, calcium, magnesium and sodium.