
Garden and Plate
The Molecular Biology of Nutrition










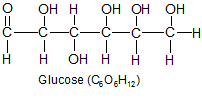 All carbohydrates and fats are made from only three elements. |
Carbon, hydrogen, and oxygen make up over 99 percent of the atoms in plants and animals. They are so widespread in organisms, they aren't considered nutrients, but are viewed as a fundamental part of every organism. Plants get these three elements from water (H2O) and carbon dioxide (CO2). Animals get them from every type of food they eat, the water they drink, and additional oxygen inhaled from the air. Carbohydrates and fats are composed of nothing but these three elements, and they form the bulk of the protein molecules as well.
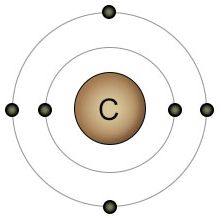
Carbon is fundamental to life. While water provides the location, carbon provides the scaffolding. Carbon's outer shell consists of four electrons and four empty spots. This gives carbon the ability to bond with up to four other atoms simultaneously, making it the most versatile atom in the biological universe. It can form a chain of carbon atoms of any length. After the carbon chain is formed, two additional atoms can be bonded to each carbon atom in the chain. This versatility allows for an unlimited number of possible configurations. These carbon chains form the backbone of many biological molecules, including carbohydrates, fats, and protein.
|
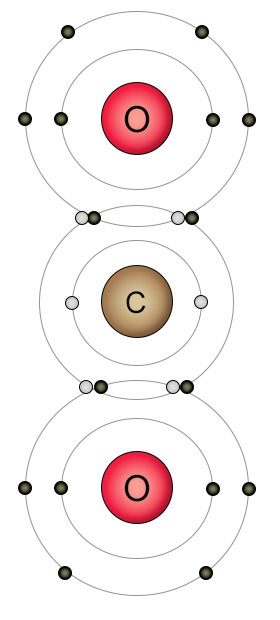
Plants get their carbon from carbon dioxide molecules in the atmosphere. Carbon atoms form double bonds with oxygen atoms because they are both looking to fill their outer shells. Two oxygen atoms fill the outer shell of one carbon atom while the carbon atom simultaneously fills the outer shells of both oxygen atoms. The result is a molecule of carbon dioxide, where all of the atoms have full shells.
There's just one problem. The double bonds formed between the carbon and oxygen atoms in carbon dioxide are strong. The plants had to develop a special process that breaks the bonds apart before they can use the carbon. The process is called the Calvin cycle, and is part of photosynthesis. The double bonds are broken and the carbon is reorganized into single bond configurations with oxygen and hydrogen atoms. These new single bond configurations (CH2O) are the building blocks of all carbohydrates.
Animals can't do photosynthesis, so they're unable to break down carbon dioxide. Animals get all of their carbon from eating plants, or from eating other animals that ate plants. It doesn't matter what the animals eat, since all plants and animals contain lots of available carbon inside their proteins, fats and carbohydrates. The only way to keep carbon out of your diet is to stop eating entirely. Fortunately, you don't have to worry about eating too much of it. Unlike many of the other elements, carbon isn't toxic no matter how much of it you eat.
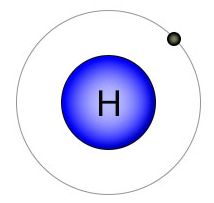
Hydrogen is the placeholder of molecular biology. With only one electron orbiting a single proton, it's small size makes it perfect for this role. It's single shell can hold no more than two electrons, so it's looking to either gain or lose an electron. Hydrogen is always available to fulfill it's placeholder role because biological processes take place in water, and water gives up it's hydrogen atoms when they are needed by other elements. By filling empty slots in the outer shell of elements like carbon and nitrogen, hydrogen keeps them in a molecular state where they are available for biologically favorable chemical reactions later on.
For example, hydrogen atoms are quick to fill the empty spots in carbon's outer shell during the Calvin cycle. This keeps carbon from re-establishing double bonds with oxygen while the precursors of carbohydrate molecules are being created. Also, when carbon forms the backbone of a fat molecule, hydrogen can occupy the remaining empty spots in carbon's outer shell before other molecules (like sulphur) can get there. Sulphur atoms make fats rancid by changing their chemical structure so that they no longer have any nutritional value. That's why manufacturers of food products saturate (fill) those empty spots in unsaturated fats with hydrogen atoms.
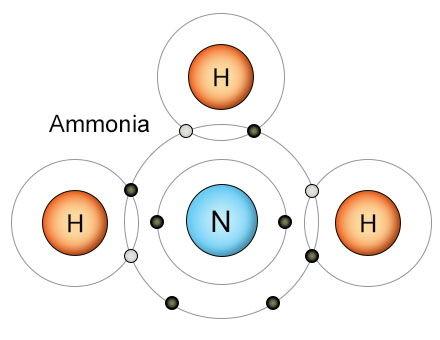
Hydrogen also plays an important role in the Nitrogen Cycle. Three hydrogen atoms fill the outer shell of a nitrogen atom, creating an ammonia (NH3) molecule. This keeps nitrogen atoms from re-combining into the triple-bonded N2 molecules that only a few kinds of bacteria can break apart. Once the nitrogen atom is safely combined with hydrogen, the resulting ammonia molecule is then converted into an ammonium ion (NH4+). As part of an ammonium ion, the nitrogen can be absorbed by plant roots. That's how hydrogen is used by bacteria to make nitrogen biologically available to plants.
Hydrogen content in food is virtually universal, since it's not only a component of every fat, protein, and carbohydrate, but is also a major part of water. You can't consume toxic levels of hydrogen. The body will just combine it with oxygen into water, and urinate it out of your body.
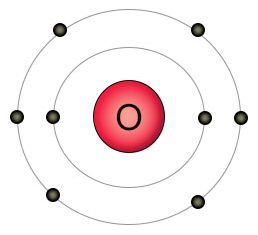
Oxygen has eight protons and eight electrons. It has six electrons in it's outer shell, and wants two more. That's why it likes to form a double bond with two of a carbon atom's empty spots to form carbon dioxide (CO2), or single bonds with two hydrogen atoms to form water (H2O). When it can't find other kinds of atoms to bond with, individual oxygen atoms will form double bonds with each other, creating O2 molecules.
Plants get their oxygen from water and carbon dioxide. When they convert water and carbon dioxide into carbohydrates, they use all of the carbon and hydrogen, but only a third of the oxygen. The extra oxygen is released into the atmosphere by the plants as waste. The release of oxygen by plants as waste doesn't mean it's toxic for plants. It just means that they take in more oxygen than they can use. As a component of fats and carbohydrates, oxygen is an essential nutrient for plants.
Animals have the opposite problem. While they get some oxygen from the food and water they consume, they don't get enough. To make up the difference, they also get oxygen from the O2 molecules they inhale from the atmosphere. These are the same oxygen molecules that were released into the atmosphere by the plants. Animals use the extra oxygen to convert carbohydrates back into carbon dioxide and water, in a process called respiration. We then exhale the carbon dioxide, which is taken up by the plants, and the cycle repeats.
Oxygen is also important because it has the highest electronegativity rating of all twenty one of the essential elements. This means that it's nucleus has a stronger pull on electrons than any other biological element. This makes it a key player in the storage and release of energy, since the change in energy levels between the oxygen and carbon bonds in carbon dioxide vs carbohydrates is where most of the free energy comes from. That's why the process that releases energy is called oxidation/reduction.
Macro means large, and these three nutrients are needed in larger amounts than any of the others (carbon, hydrogen, and oxygen are needed in larger amounts, but are not considered nutrients). Plants absorb nitrogen, phosphorus, and potassium (along with the other nutrients) from the soil, while animals get them from the foods we eat. To make sure the soil remains nutritious, these (and the other) nutrients must be added back into the soil as the plants use them up. Nature does this naturally, through composting, and we can do the same by adding compost to our gardens. We can also add chemical fertilizers, which introduce these nutrients in high concentrations and a readily available form.
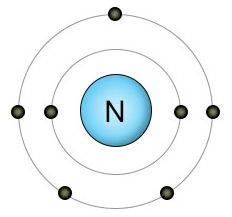
Nitrogen has seven protons and seven electrons. Two of these electrons fill it's inner shell, while the remaining five are located in it's outer shell. Since nitrogen's outer shell can hold up to eight electrons, it's looking for three more. If it finds another free nitrogen atom, they fill each other's three empty spots by forming a very strong triple bond, creating an N2 molecule. N2 molecules make up 78 percent of the atmosphere.
You might think that plants would be able to get all the nitrogen they need directly from the atmosphere. After all, that's where they get their carbon. In this case, however, neither plants nor animals are able to break apart the strong triple bonds between the nitrogen atoms in the N2 molecule. Until these bonds are broken, nitrogen isn't able to combine with other elements, and has no nutritional value.
The triple bonds of the N2 gas molecule must be broken apart before the nitrogen is available for nutritional uses.
The individual nitrogen atoms can then be kept apart by combining them with hydrogen atoms, which produce ammonium ions. They can also be kept apart by combining them with oxygen atoms, which create nitrates.
Ammonium ions (left) can be mixed together with nitrates (right) to create a fertilizer called ammonium nitrate. This chemical fertilizer is used to add nitrogen to crops.
Plants get around this inability to directly break apart (N2) molecules by aquiring their nitrogen in several secondhand ways. The first is through the action of lightning, which breaks apart N2 molecules in the atmosphere. The "fixed" nitrogen is then deposited on plant foliage by falling rain, and the plants absorb it through their leaves. Plants can also absorb nitrates (created by geologic activity), from the soil. Compost also returns nitrogen to the soil. Some kinds of bacteria are able to break apart the N2 molecules from the atmosphere, making them available to plant roots in the form of nitrogen-containing ammonium ions. These chemical transformations are part of the Nitrogen Cycle, where nitrogen moves from the atmosphere and soil, into the biosphere, and back out again.
One additional way in which fixed nitrogen is made available to plants is through the use of chemical fertilizers. The nitrogen is fixed in factories and combined with hydrogen and oxygen into ammonium nitrate, which can be added to the soil. Chemical fertilizers have their pros and cons, and are an alternative to using natural fertilizers like compost and manure. See the article on fertilizer for more details.
Nitrogen is an essential nutrient for both plants and animals because it's included in all twenty one of the amino acids that are used in the creation of proteins. It's also a structural component of ATP and NADPH (the energy carriers), and of the nucleotides, which are the building blocks of the nucleic acids (DNA and RNA). Because nitrogen is found in protein, but not carbohydrate or fat, foods that are high in protein have more nitrogen content than foods that are high in carbohydrate and fat. Nitrogen is higher in meats than it is in plants, and higher in green leafy plants than it is in starchy plants like corn and potatoes.
Most of a plant's nitrogen content is in it's leaves, because that's where most of the protein is. Chlorophyll molecules, which play a central role in photosynthesis, use lots of nitrogen. That's why adding nitrogen to the garden increases plant growth, especially in the green, chlorophyll packed leaves. Plants don't need nearly as much nitrogen for building branches or producing fruit, since these are mostly constructed out of carbohydrates, which don't contain any nitrogen.
Animals don't have to depend on lightning or ammonium ions for their nitrogen. They get all the fixed nitrogen they need from their food. Fortunately for us, the plants do all of the dirty work. By the time we consume the nitrogen in our food, it's already been re-packaged into either an amine group inside an amino acid, or into the base of a nucleotide in nucleic acid. Nitrogen plays a vital role in the structure of our genetic code, and is a necessary component of every protein in every living creature. It's easy to see why it's an essential nutrient.
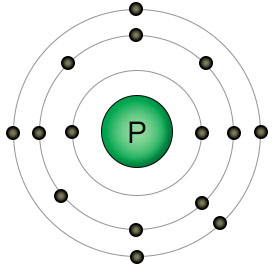
Phosphorus has fifteen protons and fifteen electrons. It has five electrons in it's outer shell, which is theoretically able to hold up to eighteen electrons. Since it has only five outer electrons, it can form a maximum of five covalent bonds with other atoms.
Phosphorus doesn't exist in the atmosphere, so plants aquire it entirely from the soil. It's highly reactive with other elements, so it's seldom found in nature as elemental phosphorus. It combines with other elements into molecules called phosphates.
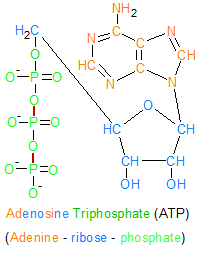
This ATP molecule contains three phosphate groups that are chemically bound to each other. Hydrolysis is used to sever the bonds between the phosphate groups, which then releases the energy required for biological processes. This transforms ATP into two molecules; inorganic phosphate and ADP. |
Organic phosphate (phosphate molecules that also contain carbon) can't be absorbed by plant roots. They have to be broken down into inorganic phosphate by bacteria first. Plants can absorb inorganic phosphate, but have a difficult time of it, since it doesn't dissolve easily in water. Most inorganic phosphate is absorbed by plant roots in the form of two molecular ions. HPO4- ions are more readily available in alkaline soils (when the PH is over 7.0), and H2PO4- ions are more readily available in acid soils (when the PH is under 7.0).
The most biologically useful form of phosphate is a molecule called inorganic phosphate (PO43-). In this molecule, the phosphorus atom forms single bonds with three oxygen atoms, and a double bond with a fourth oxygen atom. This arrangement places all five of phosphorus' outer electrons in covalent bonds.
Small amounts of inorganic phosphate are made available in the soil through the decomposition of rocks, but this is a slow process that is quickly depleted by the needs of the plants. Most inorganic phosphate is naturally available to plants from decomposed organic (plant or animal) materials like compost. The phosphates in these materials are mostly organic, but are broken down over time into an inorganic form by microorganisms, in a process called mineralization.
In the garden, where plants are routinely removed from the soil, phosphates must be added back in. This can be done with compost and manure, which release the inorganic phosphates slowly over time, or with fertilizers that release it quickly. Ammonium phosphate is a popular chemical fertilizer that adds both nitrogen and phosphate to the soil in large, readily available amounts. I prefer using compost, with it's trace elements and timed release. Compost helps with soil aeration, feeds the microorganisms, and adds many other elements to the soil in addition to nitrogen and phosphorus. Ammonium phosphate does none of these things.
Animals get their phosphates from the food they eat. Phosphates are present in the cells of all plants and animals, so they are available in a wide variety of foods. Phosphates are especially rich in foods that are high in protein, including meats, nuts, eggs, and whole grains. Because phosphates are widely available and easily absorbed by the intestine, it's easy to get more than you need. The body compensates for this by sending any extra phosphates out of the body through urination.
Phosphorus fills many biological roles. Eighty-five percent of the phosphorus in the body is bound up with calcium in the bones and teeth as hydroxyapatite, a member of the calcium phosphate family of compounds.
Inorganic phosphate (PO43-) turns up in several places in the cell. It chemically reacts with ADP, forming the ATP (energy carrier) molecule, and is a structural component in the backbone of DNA and RNA. It's also part of the phospholipid molecules that serve as the construction material for cell membranes.
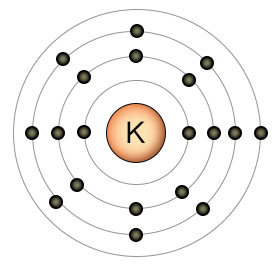
Potassium (P) has an atomic number of 19. It has only one electron in it's outer shell, so it's looking to give it away. Potassium shares many similar qualities with sodium. While sodium has eleven protons and electrons, compared to potassium's nineteen, they both have only one electron in their outer shells. Sodium has three shells, while potassium has four. They are both chemically reactive with water, and they both have nutritional value as positive ions. Despite their similarities, sodium and potassium are different chemical elements, so they can and are used against each other by the cell for purposes of diffusion through concentration differences.
Potassium is an essential nutrient for both plants and animals, but is especially important in plants, where sodium plays a minor (non-essential) role. That's why fresh fruit and vegetables are low in sodium and high in potassium, compared to animal products like meat. Potassium is available in almost all plant foods, but is especially high in foods like green leafy vegetables, whole grains, almonds, potatoes, banana's, avacados, and soybeans.
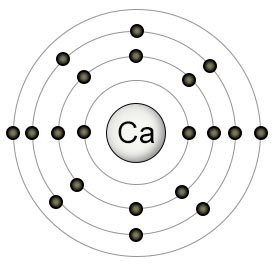
Calcium (Ca) has twenty protons and twenty electrons. It has two electrons in it's valence (4th) shell, which can hold a maximum of 32 electrons. It's 3rd shell (which can contain up to 18 electrons), is partially filled, containing just 8 electrons. Due to the quirks of the suborbitals in the 3rd and 4th shells, calcium fills the first suborbital in the 4th shell (which can contain up to two electrons), before it fills the remaining suborbitals in it's 3rd shell.
Calcium likes to give up it's two valence electrons to other atoms, converting itself into a Ca2+ ion. As a positively charged ion, Ca2+ is able to form salts with negatively charged ions. It can also form lesser bonds with organic particles in the soil that are not ions, but still have some negative charge due to their molecular structure.
When calcium ions form bonds with other substances, the Ca2+ can't be absorbed by plant roots. Fortunately, most of these bonds are water soluble, and the Ca2+ ions are absorbed into the roots along with the water. Once inside a plant, calcium ions are less mobile on their own than other nutrients, and are largely dependent upon the flow of water throughout the plant for their own distribution.
Plants don't have bones like animals do, but calcium still plays an important role in a plant's physical support. Ca2+ forms ionic bonds with pectin, creating a salt called calcium pectate. This combination of calcium and pectin provides structure and rigidity to the cell walls of plants. Animals don't use cell walls, which is why meat isn't crunchy, like apples and carrots. Plants also use calcium to help regulate the absorption of other nutrients.
In animals, most Ca2+ is used in combination with phosphate groups (PO4) to create calcium phosphate salts. One member in this family of salts, hydroxyapatite [Ca5(PO4)3(OH)], is the main structural component of bones and teeth. Animals also use calcium ions in muscle contractions and cell signaling. Both plants and animals depend on calcium's contribution to cell division.
Because calcium is a component of the cell walls of plants, it's found in a wide variety of plant foods. In non-plant foods, it's especially concentrated in dairy products. Bone meal is also a good source of calcium for both plants and animals.
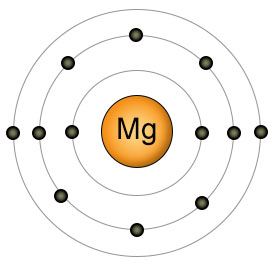
Magnesium (Mg) has twelve protons and twelve electrons. It has two electrons in it's valence (3rd) shell. It's highly reactive, so it's not found in it's elemental form in nature. Like calcium, it likes to get rid of it's two outer electrons, becoming Mg2+. Also like calcium, Magnesium must be aborbed by plants in it's individual ionic form (Mg2+). Plants can't absorb it when it's bound to other substances.
Magnesium ions perform many essential roles in both plants and animals. The magnesium ion is involved in the function of over 300 enzymes, including the ones that add and remove phosphate groups from the ATP molecule. The enzyme that stitches together nucleotides to form DNA and the enzyme that stitches amino acids together to form protein, won't function until magnesium ions bind to them. Magnesium ions also form the central point of the chlorophyll molecule used by plants to create energy.
Due to it's central role in chlorophyll molecules, it comes as no surprise that green leafy vegetables are a good food source of magnesium. Nuts and cereals are also especially high in magnesium, but it can be found at some level in almost any food. Deficiencies in magnesium are rare, and consuming high levels is rarely toxic (though still possible), since excess amounts are removed by the kidneys.
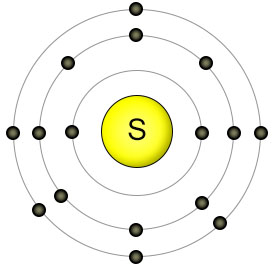
Sulphur has an atomic number of sixteen, with six electrons in it's outer (3rd) shell. When combined with other sulphur atoms, they form single covalent bonds, creating an eight-atom ring-shaped molecule called octasulphur. The octasulphur molecules then join together to form sulphur crystals. In this form, sulphur is a bright yellow solid at room temperature. Sulphur atoms can both donate and recieve electrons with other elements (depending on the element), which adds to their usefulness.
Sulphur bound to itself forms non-charged molecules. Water can't dissolve non-charged molecules, so they can't be absorbed by plant roots. Instead, plants take up sulphur from the soil in the form of sulphate molecules (SO42-). In this form the sulphur atom is covalently bound to four oxygen atoms, creating a molecule that has ionic properties (a double negative charge), so it's soluble in water.
Unfortunately, sulphur isn't biologically useful when bound to these oxygen atoms, and has to go through some chemical reactions that end up with most of the sulphur being used in the creation of two amino acids (cysteine and methionine). These amino acids perform critical roles in the structures of countless proteins, which makes sulphur an essential nutrient for both plants and animals. Cysteine amino acids in the protein keratin form strong, relatively rigid S-S (sulphur-to sulphur) covelent bonds that are used repeatedly in hair and feathers to give them their strength and durability. Keratin is also used in the construction of bones, skin, cartilage and tendons.
Since sulphur is part of the structure of two widely used amino acids, it's found in all protein-rich foods. It's found in especially large amounts in bird eggs, since chicks need lots of it to develop feathers. Once the S-S bonds are formed to create hair or feathers, they cannot be broken apart by our digestive system, which is why we don't eat them.
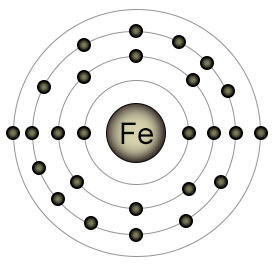
Iron (Fe) has an atomic number of twenty six. It has two electrons in it's outer (4th) shell. It's 3rd shell is partially full, with six (out of a possible ten) electrons. This is because the S suborbital of the 4th shell fills before the D suborbital of the 3rd shell. Elemental iron is reactive, and is usually found in one of two ionic forms (also called oxidative states); Fe2+ (with it's 4th shell emptied), or Fe3+ (with an additional lost electron, this time from the D orbital of the 3rd shell).
Plants have difficulty absorbing Fe3+ from the soil, especially in soil with neutral to high PH levels. They have an easier time absorbing Fe2+. Plant roots can absorb Fe3+ by adding an electron to it first, which converts it to Fe2+. Once inside the plant, iron performs an essential role as part of a protein that's involved in the creation of chlorophyll. Even when it's not absorbed by the roots, iron can still be beneficial to plants. Iron ions are a component of the nitrogenase proteins that perform nitrogen fixation, which makes nitrogen available to the plants.
Animals also find iron absorption a difficult process. Absorption takes place in the upper part of the small intestine, but only a small portion (perhaps 3%) of what's contained in the food makes it into the bloodstream. Once it's absorbed, the body re-uses it, and has almost no mechanisms for getting rid of excess amounts. The body's inability to excrete excess iron can make it toxic in large amounts. Most iron is stored in the body in the blood, liver, spleen, and bone marrow, and is always associated with proteins, once inside. For animals, iron's most important contribution is it's ability (as part of the protein hemoglobin) to transfer oxygen from the lungs to the cells where it's needed. Myoglobin, a protein similar to hemoglobin, uses iron in a similar manner to make oxygen available to muscle cells.
Foods high in iron include the liver, because that's one of the places that iron is stored in the body. Green leafy vegetables are also high in iron, because it's used to create chlorophyll. The greener the leaves, the higher the iron concentrations. This makes dark green leaves like spinach especially high in iron.
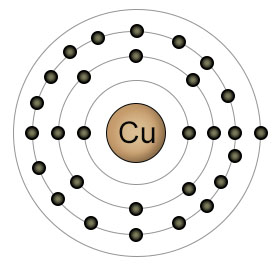
Copper has an atomic number of twenty nine. It has a completely filled 3rd shell, and just one electron in it's 4th (outer) shell. Copper is usually found in one of two ionic forms. Cu+, which has lost it's single 4th shell electron, and Cu2+, which has lost an additional electron, this time from it's 3rd shell. It can easily convert between these two ionic forms, both in the soil, and inside plants and animals.
Copper is one of the most difficult elements for plant roots to absorb, since it likes to bind with organic molecules in the soil. Plant uptake of copper increases when the PH level of the soil is below seven, because the low PH level increases the amount of Cu2+ relative to Cu+. Once inside the plant, copper plays an essential role in photosynthesis. It's a component of the protein plastocyanin, which takes part in the electron transfers that make photosynthesis possible.
In animals, copper has a role to play in both neurological function and the maintenance of the immune system. Copper is easily converted back and forth between it's two ionic forms (Cu+and Cu2+) in both plants and animals, which makes it an important player in reactions that require the transfer of an electron. Copper is a component of some of the proteins that are involved in the processing of oxygen.
Foods that contain the highest concentrations of copper include shellfish, organ meats, green leafy vegetables, nuts and seeds. Consuming too much copper can be toxic for both plants and animals, but copper is available in foods in such small amounts that toxic levels are virtually unknown.
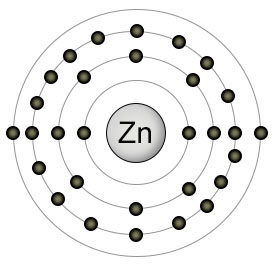
Zinc has an atomic number of thirty. It has a full 3rd shell, and two electrons in it's 4th (outer) shell. It's most commonly found in it's ionic state (Zn2+), since it likes to empty it's 4th shell of it's two outer electrons. This ionic condition, however, sets it's 4th shell up to recieve four pair of electrons from four other atoms, usually nitrogen, sulphur, or oxygen. These four coordinated covalent bonds position zinc in the active site of many enzymes, where it plays both catalytic and structural roles.
Plants usually absorb zinc from the soil as Zn2+, but can also absorb it when it's bound to other substances like ZnOH+. The way in which zinc forms it's coordinated bonds with other atoms in a protein gives the protein the ability to change shape easily. This is why zinc plays a structural role in so many proteins. Both plants and animals use zinc in the enzymes that perform DNA replication and transcription.
Zinc moves through the bloodstream by binding to albumin and transferrin molecules. The enzyme carbonic anhydrase, which converts bicarbonate into carbon dioxide (so it can be exhaled from the lungs), uses zinc as part of it's structure. Zinc is also included in the structure of insulin, as well as the enzyme that breaks down alchohol.
Since zinc is a component of so many different proteins, it can be found in significant amounts in all high protein foods, especially red meat. It can also be found in high concentrations in oysters, lobster, wheat germ, and almonds. Some seeds, such as pumpkin and sunflower, are also good sources.
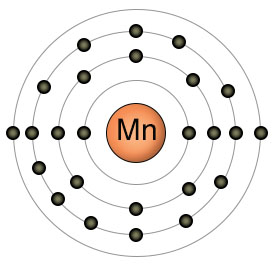
Manganese has an atomic number of twenty five. It has a partially filled 3rd shell with thirteen electrons, and two electrons in it's 4th (outer) shell. Manganese is in it's most stable (and biologically useful) state as Mn2+, when it has given up both of the electrons in it's 4th shell.
Plants absorb manganese in the form of Mn2+, which becomes more available to the roots as the PH levels decrease. High PH levels cause Mn2+ to bind to other substances, decreasing it's availability.
Plants use Manganese in many processes, usually as an activator of enzymes. It plays an essential role in photosynthesis, taking part in the photosynthetic electron transport chain.
Animals don't perform photosynthesis, but they still require manganese. Manganese is involved in bone formation, and a deficiency can cause abnormalities in skeletal development. Most manganese is stored in the bones, but some can also be found in the liver, kidneys and brain.
Manganese can be found in a wide variety of foods, but is especially high in oatmeal, pecans, whole wheat, beans, and pineapple.
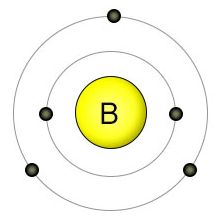
Boron has an atomic number of five, making it one of the smaller biological elements. It has three electrons in it's 2nd (outer) shell, which can hold a maximum of eight electrons. This allows it to form up to three covalent bonds. It's the only non-metal element among the micro-nutrients.
Boron is usually taken up by plant roots in the form of boric acid (H3BO3), where it's passively brought into the roots along with water. Boron deficiency in plants is more common than with any other known micronutrient. Since boron uptake is directly related to water uptake, the amount of boron that a plant gets is partly dependent on how much water the plant uses. In addition, since plants take it up in the form of boric acid, boron becomes less available as the PH of the soil rises.
On the other hand, it's easy for plants to aquire toxic levels of boron. Since boric acid is taken up automatically with water, plants have no way to regulate it's absorption. This means that keeping levels of boric acid in the soil between .3 and 1 parts per million (the ideal range) is important. Adjusting the PH level is one way to control this. If boric acid levels in the soil get too high, they can also be lowered the way that sodium concentrations are lowered: by flushing out the soil with lots of water.
Boron is mostly used by plants in the construction of cell walls, where it's involved in the binding of pectins. Boron is also involved in flowering, seed formation, and the movement of plant hormones. All plant-based foods contain trace amounts of boron, so we consume it from a wide variety of sources. While essential for plants, we haven't yet found a place for it in animal nutrition.
Molybdenum has an atomic number of forty two, making it the 2nd largest nutritional element. Iodine is the largest. Molybdenum has thirteen electrons in it's partially filled 4th shell, and one electron in it's 5th (outer) shell. It's most stable when it loses either four or six electrons (Mo4+ or Mo6+). This lets it shed it's 5th (outer) shell, and sets up it's 4th shell as the valence shell, with the ability to form multiple covalent bonds with other atoms.
Plants take up molybdenum when it's bound with oxygen, as a molybdate ion (MoO42-). Molybdenum is the only micronutrient that becomes more available to plants at higher PH levels. Molybdenum's main nutritional value comes from it's use in the active centers of many different enzymes. It's most useful to plants as an essential component of the enzyme nitrate reductase, which breaks down nitrates (NO3-), making the nitrogen available to the plant for biological processes. Nitrate reductase is one of the enzymes that requires molybdenum in it's active site.
Another one of these molybdenum-dependent enzymes provides nitrogen to the roots from outside the plant. Certain types of symbiotic bacteria fix nitrogen, and then supply the fixed nitrogen to the plant roots for absorption. Bacteria can perform this service because they contain the nitrogenase enzyme, which converts atmospheric nitrogen into nitrogen-containing ammonium ions for the plants. The nitrogenase enzyme, which plants don't have, depends on the presence of molybdenum in it's active site.
Animals don't have the nitrogenase enzyme either, but they use other molybdenum-containing enzymes. Two examples are xanthine oxidase, which is used to release iron reserves from the liver, and aldehyde oxidase, which is involved in the oxidation of fats. Molybdenum is also a component of tooth enamel.
As in plants, molybdenum is transported throughout our bodies in the form of MoO42-, and is stored in the bones, liver, and kidneys. Molybdenum is contained in many foods in trace amounts. It's highest concentrations are found in eggs, legumes, liver, and cereal grains.
Chlorine has an atomic number of seventeen. It has seven electrons in it's outer shell, and is looking for an eighth. Chlorine (like many of the elements), is a toxic substance in it's atomic form. It derives it's nutritional value from it's ionic incarnation as chloride. It plays an important role in digestion as a component of hydrochloric acid. Chloride is a negative ion, so it easily forms ionic bonds with sodium atoms after sodium gives up it's outer electron to become a positive ion. Sodium and chloride usually arrive on our plates together as table salt, so I examine them together in more detail on The Sodium/Chlorine Reaction page.
In addition to the other elements on this page, which are essential nutrients for both plants and animals, there are five more nutrients that are essential for animals, but not for the plants. Some of these (like sodium) are used by plants if available, but aren't essential for their survival. Others (like iodine) aren't used by plants at all, even though some plants may contain them.
Sodium has an atomic number of eleven. It has one electron in it's outer shell, which it will donate to any passing element that needs an extra electron. This makes sodium a reactive element. Sodium is the only element that has it's own taste receptor built into our tongues, which gives you an idea of it's importance in nutrition. It plays a minor (non-essential) nutritional role in most plants, but plays a major role in animal nutrition. It usually (but not always) arrives on our plates combined with chloride as table salt. Sodium plays an essential role in many biological processes, which I examine in detail on the Sodium/Chlorine Reaction and Diffusion pages.
Cobalt has an atomic number of twenty seven. It's nutritional value is found entirely in the role it plays as a structural component of the vitamin B12 molecule. Vitamin B12 is an essential nutrient, making cobalt an essential element for animals. Plants don't need it, and most of them (except for some ocean plants) don't contain it. Cobalt forms ionic bonds with other elements, so it's usually found in nature bound up in salt compounds (not table salt). Bacteria in the guts of ruminant animals can produce Vitamin B12, but humans can't create it in their bodies. We must get it in our food.
Cobalt by itself has no nutritional value. Vitamin B12 (and the cobalt it contains) is found in it's highest concentrations in the muscle tissues, as well as several organs in the body. The liver and kidney have especially high concentrations of vitamin B12. If you're looking for foods that contain vitamin B12, pick animal-based products like meat, organs, eggs and milk. You won't find it in your salad.
Iodine (I) is the largest biologically useful atom in plants and animals. It has 53 protons and 53 electrons. It organizes it's 53 electrons into five electron shells. The inner four shells are full, but it's outer (fifth) shell (which can contain up to 50 electrons), has only seven electrons. The fifth shell divides it's electrons into two suborbitals, starting with "s", which contains a maximum of two electrons, then "p", which can contain a maximum of six electrons. The other fifth shell suborbitals are empty. With seven electrons in iodine's outer shell, it's "s" suborbital is full, and it's "p" suborbital (which contains five electrons) needs a sixth to be full.

Iodine becomes the iodide (I-) ion by taking an electron from another element, which fills it's "p" suborbital. As a newly created ion, it then forms a salt by establishing an ionic bond with the other element, which became a positive ion when it gave iodine it's electron. If the other element was potassium, it combines with the potassium ion (K+) to form a salt called potassium iodide (KI). If the other element was sodium, it combines with the sodium ion (Na+) to form a salt called sodium iodide (NaI). Iodine can also form salts with other positive ions. As iodine, it's not very soluble in water. But in it's ionic form (iodide, I-), it becomes much more soluble in water.
The salt that contains the iodide is dissolved in the body, and the iodide is collected by the thyroid gland. The thyroid gland then uses the iodide to create hormones. One of the hormones is called thyroxine (T4), which contains four iodine atoms (the iodide oxidizes back into iodine when it forms a covalent bond with a carbon atom to become part of the thyroxine molecule). The thyroid gland releases T4 into the bloodstream, where it's taken up by the cells. Once it's in the cells, it's converted by the deiodinase enzyme into the hormone triiodothyronine (T3) by removing one of the iodine atoms. As mentioned in the article on selenium (which is a necessary component of the deiodinase enzyme that converts T4 hormones to T3), these iodine-containing hormones play a vital role in many biological processes in the body, including overall growth and development (including brain development), and the regulation of the heart rate and body temperature. They also control the rate at which the cells use oxygen, thereby regulating the metabolism.
Iodine is found in many foods, but usually not in high enough amounts to meet our needs. It's found in higher concentrations in foods from the sea (like fish and kelp), than in land-based plants, but can also be found in significant amounts in dairy products and eggs. Plants will absorb it from the soil if it's available, but many soils are iodine poor. If this is the case, people may not be getting enough iodine in their diets. Iodine deficiencies are the most common cause of goitre, a swelling of the thyroid gland. Since iodine deficiencies are also the leading preventable cause of mental retardation, many governments require that it be added to table salt. Iodized salt is usually table salt (NaCl) sprayed with potassium iodate (KIO3). Potassium iodate is used instead of potassium iodide to iodize salt, because it has a longer shelf life.
Iodine, in the form of potassium iodide, is used to prevent radiation poisoning when people are exposed to nuclear fallout (like when a nuclear power plant has a containment breach). Nuclear fallout contains a radioactive isotope of iodine called iodine-131. Since the thyroid gland stores iodine so it can produce hormones, the iodine-131 collects there, exposing the body to radiation. To prevent this, people are given potassium iodide. Potassium iodide contains iodine-127, the only stable (non-radioactive) isotope of iodine. The thyroid can only absorb so much iodine. If the iodine-127 in potassium iodide gets there first, there won't be room for the radioactive iodine-131. That's how iodine tablets prevent radiation poisoning.
Isotopes are variations of the same atom, with different numbers of neutrons in their centers. An iodine atom always has 53 protons in it's nucleus. That's what makes it iodine. But in addition to the protons, it also contains uncharged particles called neutrons. Neutrons have the same mass as protons, but protons have a positive charge. To distinguish isotopes from each other, the total number of protons and neutrons in the nucleus are added to the atom's name. In the case of iodine-131, there are 78 neutrons (53 protons + 78 neutrons = 131). The problem with iodine-131 is that it's unstable. Neutrons usually stabilize the nucleus, but some proton/neutron combinations are unstable. When things break down in the nucleus, particles and energy are released (radiated), and cause damage to cell structures, causing radiation poisoning.
Chromium (Cr) has 24 protons and 24 electrons, and is usually found in one of two states: trivalent (chromium 3+) and hexavalent (chromium 6+). The hexavalent form is toxic in any amount, but the trivalent form is an essential nutrient in trace amounts. Chromium works with insulin to increase it's effectiveness in the management of carbohydrate, fat, and protein levels in the body. Chromium helps insulin move glucose into the cells, and assists enzymes in the breakdown of glucose once inside.
Chromium is widely distributed throughout the food supply in low amounts, but can be found in higher than average levels in meat and whole grains. Most fruit and vegetables have comparatively low levels, with the exception of a few items, like broccoli and grapes.
Selenium (Se) has 34 protons and 34 electrons. It's an essential micronutrient for animals, but is toxic when consumed in large amounts. Plants absorb it from the soil, but it's not essential for their survival, so it's not included on the list of essential nutrients for plants. A few plants, like some varieties of locoweed, absorb large concentrations of it as a defense mechanism. Animals that eat too many of their leaves can die from toxic levels of selenium.
Selenium is essential in trace amounts for animals (including us), because it's a necessary component in the structure of some enzymes (including Deiodinase and glutathione peroxidase) that perform important biological functions.
The deiodinase enzyme, for example, is important because it converts a hormone called thyroxine (T4) into the hormone triiodothyronine (T3). The thyroid gland produces T4, then releases it into the bloodstream. Once it arrives in the cells where it's needed, the deiodinase enzyme converts it into T3 by removing an iodine atom from the T4 molecule. The T3 hormone plays a vital role in many biological processes in the body, including overall growth and development. It also helps to regulate heart rate and body temperature. Selenium, as a component of the deiodinase enzyme, plays a vital role in the thyroid gland's production of the T3 hormone.
Selenium is also a component of glutathione peroxidase. Glutathione peroxidase refers to a family of several closely related enzymes that protect cells from oxidative damage. They do this by reducing a variety of peroxide molecules to alchohol or water. For example, glutathione peroxidase will break down hydrogen peroxide (H2O2) into water (H2O) and oxygen (O), before the hydrogen peroxide has a chance to react with (and damage) other molecules. Since it's an enzyme, glutathione peroxidase is unchanged in the reaction, and can go on to break down additional hydrogen peroxide molecules.
Selenium can be found in meat and eggs. It's found in relatively high concentrations in some sea foods like tuna and crab, and is especially high in brazil nuts. Although most plants don't require selenium for their own biological processes, many of them contain various amounts of it. Selenium can also be found in cereals and mushrooms.
Selenium's toxicity is caused by it's ability to replace sulphur as a building material in the construction of some amino acids. In high enough concentrations of selenium, a biologically significant number of amino acids will be affected. Proteins created from these selenium-containing amino acids will fold differently, causing them not to function properly. If the cell can't produce enough of the correct sulphur-containing amino acids, cellular processes will be compromised.
The Periodic Table of the Elements consists of one hundred and eighteen elements, eighty nine of which are found naturally in the physical world. Only twenty one of these elements are considered essential for life.
All twenty one of these essential elements are described individually on the left side of this page. Sixteen of these elements are considered essential for both plants and animals, and are listed below. Five additional elements aren't required by plants, but are essential for animal life. They are listed further down this column.
The 16 elements listed below are essential for both plants and animals. They are needed in different amounts, and have been sub-divided into four groups to reflect this. The four groups are described below the list.
Carbon, Hydrogen, and Oxygen are needed in huge amounts, and are supplied by water and air (H2O and CO2). They are central to most molecular processes, but the bulk of them are used to create carbohydrates and build the physical structure (cellulose or wood) of the plant. Together they make up over 99 percent of the plant. They are not considered nutrients.
Nitrogen, Phosphorus, and Potassium are called the "Macro-nutrients", because they are needed in larger amounts than the others.
Calcium, Magnesium and Sulphur are called the "Secondary Nutrients" because they are needed in smaller amounts.
The remaining seven elements are called the "Micro-nutrients", because (while essential), they are needed in extremely small amounts. These include iron, copper, zinc, manganese, boron, molybdenum, and chlorine.
It should be noted here that while these are all essential nutrients, many of them are toxic in their elemental (individual atomic) state. Most of them are absorbed into the plant as molecules with significantly different chemical characteristics than the individual atoms that comprise them. In some cases the molecules dissolve in the presence of water, but even then they often separate as ions, which is the form in which they provide nutritional value. Sodium and Chlorine are a perfect example of this.
In addition to the sixteen essential elements for plants, animals need these elements:
These two "parts lists" for plants and animals include most of the essential elements, but are not necessarily comprehensive. Once you get down to the trace elements, some findings are inconclusive, and it's possible that additional elements play minor roles that haven't yet been discovered. But for our nutritional purposes, all of the major players are listed and described on this page.
Several articles on this site provide detailed descriptions of the biological processes that some of these elements are involved in.
Fertilizer is all about adding specific elements into your soil. When you buy fertilizer, it's usually labeled with a three number code, like 5-10-5. These three numbers refer to the relative concentrations of each of the three macro-nutrients listed on the left side of this page.
While nitrogen, phosphorus and potassium in chemical fertilizers are chemically identical to the same elements in compost, manure, and natural fertilizers, they are not presented to the plants within the same context. In natural substances like compost, they are bound up with other substances, and release them slowly over time. In chemical fertilizers, they are available all at once. In addition, natural fertilizers also include trace nutrients and soil amendments that are essential for the plants, while chemical fertilizers often don't.
Plants and animals evolved over millions of years in environments that offer up nutrients in the form of compost and whole foods. These nutrients are provided in the optimal concentrations and combinations through the co-evolution of plants and animals. While chemical fertilizers can be used to produce specific results in certain circumstances, they aren't the way to establish biological balance in the garden. Too much nitrogen, for example, can kill microorganisms that release nutrients into the soil, and "burn" plant roots. The plants and soil aren't able to maintain a state of balance (called homeostasis) when the nutritional composition of the soil swings wildly due to additions of chemical fertilizers.
The same principle holds true with the food we consume. The fructose in table sugar is chemically identical to the fructose in an apple. Both are converted by the kidney into glucose, an essential nutrient. An apple, however, is a better nutritional choice than a handful of sugar cubes, because the sugar cube contains nothing but fructose and glucose, while the apple contains many other nutritional elements. The apple also contains fiber, which slows down the absorption of fructose into the bloodstream to rates that the liver and pancreas can better cope with. Sugar cubes don't contain fiber.
Having said all of that, the green revolution would have been impossible without the development and widespread use of the chemical fertilizers. Starvation would be much greater (or the human population much smaller) without them. But just because producing thousands of acres of a single crop through the use of chemical fertilizers is the most cost-effective way to feed the world, it doesn't mean that the same method is the most productive (or enjoyable) way to maintain a garden.
One of the best things about growing your own food is the ability to produce much higher quality fruit and vegetables than you can get in the supermarket. While you may not notice much difference between some store-bought versus home-grown produce, the difference in flavor is huge when it comes to things like strawberries and tomatoes. If one of the reasons you garden is the higher quality of home-grown produce, why use the fertilizers that were created for large-scale agriculture when going organic works so much better in our small-scale gardens?
Our atmosphere is composed mostly of small, non-polar molecules that are bound to each other in ways that keep them from combining into larger molecules. That's why the atmosphere is a soup of gases instead of a solid or a liquid.
Nitrogen atoms combine with each other to form N2 molecules, which make up 78% of the air we breath. We can't use nitrogen in this form for nutritional purposes, since certain bacteria are the only organisms that can break it apart.
Oxygen forms bonds with itself to create O2 molecules, which make up 21% of earth's atmosphere. Most of this oxygen was put there by the plants, which released it from carbon dioxide (CO2) during photosynthesis. O2 is biologically available to animals when it's taken up by the lungs.
The last 1% of the atmosphere is made up of several trace elements that include argon, ozone, and carbon dioxide. Carbon dioxide is the most nutritionally important trace element in the atmosphere (even though it makes up only about .04% of it), because plants break it down to get at the carbon.
All of the elements are individual atoms, and are defined by the number of protons they contain. Individual atoms are the smallest items that have distinct chemical properties. Every element listed on the left side of this page breaks down into identical protons, neutrons and electrons. It's the number of protons and electrons that make them chemically distinct. If we move up one level and combine the elements into molecules, we get additional chemical properties that none of the individual elements possess.
Just as the twenty-six letters of the alphabet can be combined into a dictionary full of words, the twenty-one elements described on this page can be combined into a huge number of chemically distinct molecules. Most of these possible combinations have no nutritional value. Others, like the proteins, fats, carbohydrates and vitamins are essential for life.
Between the individual elements and the larger molecules just mentioned, there's a class of small molecules that are used over and over in biology due to their beneficial chemical properties. They may be on their own, or part of a larger molecule, but they are aptly called "functional groups" because they are defined by how the elements in them function as a group.
Most biological molecules are built around a carbon-carbon backbone. Any unoccupied spots in carbon's outer shell are usually filled by hydrogen. C-C and C-H bonds are non-polar because carbon and hydrogen have similar levels of electronegativity. When they are joined to other elements that have higher or lower electronegativity, the combinations are polar. This is where the functional groups value comes from.
Oxygen has a very high electronegativity, so it's a component of several functional groups. When oxygen combines with hydrogen, a hydroxyl (OH) group is formed, and when it combines with carbon you get a carbonyl (CO) group. Oxygen and phosphorus join together to form a phosphate (PO4-2) group. Oxygen isn't the only element that can create functional groups. When nitrogen combines with hydrogen, you get an amino group (NH2).
There are many additional functional groups. They all have different chemical qualities and react with each other in unique ways. That's what makes them functional.
For instance, the polar nature of a phosphate group makes it possible to transfer energy from one molecule to another. This typically happens during phosphorylation, when the phosphate group is transferred to or from an ATP molecule.
Amino acids are another example of how functional groups react. Amino acids are stitched together into proteins when the amino group on one side of the amino acid reacts with the carboxyl group on the other side of an adjacent amino acid. Functional groups are the molecular grease that keeps the biological machinery going.