
Garden and Plate
The Molecular Biology of Nutrition











When it comes to molecular biology, the three most important things are location, location and location. What does this have to do with water, you ask? Water is where it's at. Literally. We may not know how life first arose, but we know where it got it's start. The molecular processes that make life possible occur only in the presence of water.
When life crawled out of the oceans, it had to take plenty of water with it. The cells in our bodies are full of the stuff. But water does more than provide a location for life. It's also an active participant in many of life's molecular processes. This small molecule plays a huge role in molecular biology. Let's examine the water molecule in detail.
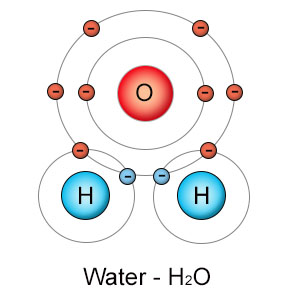
Water is composed of two hydrogen atoms that are covalently bound to an oxygen atom. Hydrogen atoms like to form a single bond, while oxygen atoms like to form two bonds. They combine as H2O, forming what would seem to be a stable water molecule. In reality, things aren't that simple.
Oxygen is more electronegative than hydrogen. When they form a covalent bond with each other, the electrons aren't shared equally. Oxygen pulls hydrogen's electron close to it's powerful (eight proton) nucleus, and far from hydrogen's weak (one proton) nucleus. Since electrons carry a negative charge, the oxygen side of the water molecule becomes more negatively charged than the hydrogen side. This is what it means to be a polar molecule. Water's polarity is especially strong because both hydrogen atoms attach to oxygen near each other (104 degrees apart), instead of on opposite sides. Water's small size, high polarity, and ability to break down into highly reactive components give it a unique combination of biological characteristics.
The sharing of electrons between hydrogen and oxygen is so unequal, hydrogen barely manages to maintain it's grip at all. This creates an unstable bond. Any transient force, including random thermal energy or the tug from another polar molecule, can cause the hydrogen atom to lose it's grip on the electron it shares with oxygen. Hydrogen is a single proton orbited by a single electron, so when hydrogen loses it's grip, it floats off as a single positively charged proton. When this happens, the water molecule splits into a positively charged hydrogen ion (H+) and a negatively charged hydroxyl (OH-) molecule.
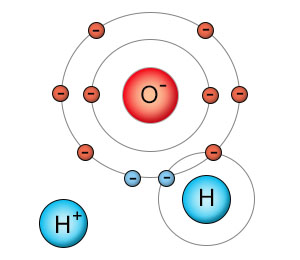
Water molecules can easily be divided into two highly reactive charged parts. When the nucleus of a hydrogen atom separates from a water molecule, you produce a hydroxyl anion (a negatively charged hydroxyl group) and a positively charged hydrogen ion. The separated hydrogen nucleus is now a bare proton, and wants to aquire two electrons to fill it's now non-existant shell. |
The separation of positively charged hydrogen ions (protons) from water molecules happens all of the time, and the relative concentrations of protons and OH- molecules to H2O reaches a state of equilibrium in pure water. The number of charged parts is very small compared to the number of intact water molecules because the hydrogen ion is quickly attracted to and re-combined with a negatively charged (OH-) molecule. Despite their relatively small numbers, the high reactivity of these broken bits of water make them key players in many biological processes.
The equilibrium becomes disrupted when other substances are added to pure water. Imbalances in the number of free protons and OH- molecules occur because some substances (acids) will donate protons to the solution, while others (alkalines) remove them. If you add an acid to water, the acid will donate protons to the solution. There won't be enough OH- molecules to combine with them to create new water molecules. The extra protons will instead attach themselves to existing water molecules, forming hydronium ions (H3O+). The hydronium ion will give up it's extra proton the first chance it gets, but in a highly acidic solution they have nowhere else to go.
Why is all of this so important in biology? Because many of the chemical reactions that take place in the body depend on the availability of both of these reactive particles. If the balance between these two substances is disturbed, one will cease to be available. It will combine itself out of exisitence when significantly outnumbered by the other. If the imbalance is too great, the solution will no longer be able to complete the biological reactions that support life. A balance must be maintained.
| 10,000,000 | pH 0 | Battery Acid |
| 1,000,000 | pH 1 | Stomach Acid |
| 100,000 | pH 2 | Vinegar |
| 10,000 | pH 3 | Grapefruit, Wine |
| 1,000 | pH 4 | Beer, Tomatoes |
| 100 | pH 5 | Cabbage, Coffee |
| 10 | pH 6 | Saliva, Egg Yolks |
| 1 | pH 7 | Pure Water |
| .1 | pH 8 | Sea Water |
| .01 | pH 9 | Baking Soda |
| .001 | pH 10 | Milk of Magnesia |
| .0001 | pH 11 | Ammonia |
| .00001 | pH 12 | Soapy Water |
| .000001 | pH 13 | Bleach |
| .0000001 | pH 14 | Drain Cleaner |
pH 0 is the most acidic. pH 14 is the most alkaline. pH 7 is considered neutral, and divides the acidic from the alkaline. Most food has a pH of 3 to 6. | ||
Maintaining a healthy balance between protons and OH- molecules is critical for both plants and animals, so a pH system has been developed to measure it. The "H" in pH refers to the hydrogen ions (protons) that are being measured. The "p" in pH is a mathematical symbol that means "the negative logarithm of". So pH refers to the number of free protons in a solution, measured on a logarithmic scale. A logarithmic scale is used so we can compare huge numerical differences in proton levels using small numbers. Since the logarithmic scale is negative, more protons correspond to a lower pH. Don't worry about the math. The important thing to know is that pure water is considered neutral, with a pH level of 7. If a solution has more protons than OH- molecules, the pH level is below 7 and the solution is acidic. If it has less protons, the pH level is over 7, and the solution is alkaline.
The pH 7 level is a good reference point for biological activity, because life evolved in water. Different biological processes, however, require different levels of pH. The bloodstream is maintained at a 7.4 pH level, which is optimal for the chemical reactions that take place there. The stomach, on the other hand, maintains the much lower pH level of 2 by injecting gastric acid into it's food. Most of the foods we eat have a pH level between 3 and 6, so the stomach must need lots of free protons when digesting it's food.
pH is important in the garden because some plants need different pH levels in their soil than others. Plants use imbalances of charged particles and nutrient concentrations to facilitate their diffusion across the soil/root barrier. Too many charged particles like OH- and H+ can interfere with that diffusion.
In the kitchen, pH is used as a preservative and a flavor enhancer. White vinegar has a pH level of 2.4, which is too low for most biological activity. This is why it can be used as a preservative when pickling food. The free protons in acidic foods are also able to enter openings in the "taste bud" cells on our tongue, which sends a signal to the brain. We experience this reaction as the sour sensation of taste.
Water routinely breaks up into H+ and OH- because it's easy to do. Hydrogen's electron is bound so tightly to oxygen that it takes very little energy to remove the hydrogen nucleus, leaving hydrogen's electron behind. But what if we want to separate the hydrogen atom completely, so it takes it's electron with it? That would require lots of energy. The oxygen atom, with it's high electronegativity, has a firm grip on hydrogen's electron. It would be like rolling a large rock up a steep hill. Why would we want to do it? Because it's a way to store energy.
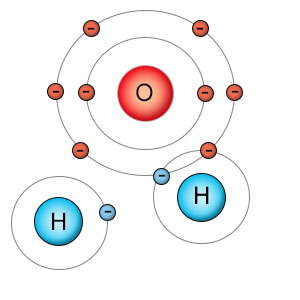
Water molecules can also be broken down into non-charged but highly reactive parts. In this case hydrogen and the hydroxyl radical are both looking to fill empty slots in their electron shells. |
Oxygen has a higher electronegativity rating than any other biologically useful element. This means it attracts electrons with more force than other atoms. Having an electron tightly bound to an oxygen atom is like having a rock at the bottom of a hill. It's already at it's lowest energy point, so you can't get anymore energy out of it. Hydrogen atoms have a much lower electronegativity rating, so when an electron transfers from a hydrogen atom to an oxygen atom (or is shared with oxygen), hydrogen's electron "rolls downhill", and energy is released. The opposite is also true. When we transfer an electron from oxygen to hydrogen, energy is used. When we separate hydrogen from oxygen, we are essentially storing energy that can be used later on. Plants take advantage of this in photosynthesis.
The reactions that separate water into (H+) and OH- particles are important for acid/alkaline reactions, but they don't store and release energy like the hydrolysis and condensation reactions that break apart and recombine the H and OH particles. Water is split into H and OH particles in a reaction called hydrolysis. Hydrolysis is typically used by an enzyme in a reaction that splits another molecule in half. To keep the two ends of the newly split molecule from recombining, H and OH are added to the ends of the two new molecules. Condensation does just the opposite. It takes place when H and OH are removed from the ends of two molecules as they are joined together. Keeping plenty of H and OH particles in the cell is important to make sure these reactions take place as needed.
Water performs lots of important biological functions when you break it up into little bits. But water also has a few tricks up it's sleeve when you leave it in one piece. Water's small size and high polarity make it the perfect solvent for other polar molecules. It's small size enables it to wiggle in between other polar molecules, disrupting the hydrogen bonds that hold them together. It's negative side attracts the positive side of other polar molecules, while it's positive side attracts their negative side. Since water molecules have a higher polarity than other molecules, they can dissolve many other substances. Even those that are held together by ionic bonds, like salt.
Water isn't really a universal solvent, because it doesn't dissolve covalent bonds, and doesn't separate non-polar molecules from each other. That's a good thing. If water was a universal solvent, nothing would hold it. We're mostly made of water, so we'd dissolve into a puddle.
Water separates polar molecules by separating their negative and positive sides. Non-polar molecules don't have negative and positive sides, so they aren't dissolved by water. Instead, water molecules are attracted to each other, causing them to cluster together. This pushes non-polar molecules into clusters of their own. This is why oil and water don't mix. Oils are non-polar molecules.
Heat is caused by the motion of atoms within a substance. Increased movement causes a higher temperature. Just as you can fit more couples on a dance floor during a slow dance than a fast dance, you can fit more molecules into a given area when they are cold than when they are hot. This explains phenomenon as varied as a pot boiling over on the stove and the wind blowing through your backyard (high density cold fronts flow into low density warm areas).
Water molecules do the same thing through-out most of their temperature range, with one very important exception. Due to the unique shape and polarity of the water molecule, water expands as it solidifies into ice. This is because the water molecules align into an open lattice structure that's less dense than when they are in liquid form. If water didn't have this property, ice would sink instead of float. Oceans and lakes would freeze from the bottom up instead of freezing over.
Water's ability to expand as it freezes has another important consequence. Water uses it's unique characteristics as a wedge. It fills small cracks in rocks when it's a liquid, then expands as it freezes, breaking the rocks into smaller pieces. This is an important addition to erosion's toolkit, which helps it to add minerals to the soil.

Milk is composed mostly of water, so it expands when it freezes. Milk placed in this container expands out of the top and cracks the glass as it freezes.
When fruit contains a high number of acidic molecules it has a low pH level, and tends to be sour. Many plants raise the acidic content of their fruit to make it unpalatable until the seeds are mature. These grapes are a perfect example. They were green and very sour just a few weeks earlier. Now they are red and sweet. Enzymes in the grapes break down the acidic substances and increase the sugar content as they ripen.

Acidic foods taste sour because positively charged protons diffuse from acidic (proton donating) molecules into the sour-detecting cells on your tongue. The diffusion of these protons creates a disruption in the voltage levels that normally exist across the cell's membrane. This causes the cell to release a neurotransmitter that sends a "sour" signal to the brain. For more details on how this takes place, see the article that describes how cells use diffusion to transmit signals.

Photosynthesis powers the biological world, and plants use water to make it happen. How do plants do this? They push electrons in the water molecules into higher orbitals to create carbohydrates.
Energy from the sun is used to separate water molecules into hydrogen and oxygen. The newly freed hydrogen atom is then bound to a carbon atom that was derived from the splitting of carbon dioxide. Carbon atoms have a lower electronegativity than oxygen, so the electron of a hydrogen atom is energetically "uphill" when shared with a carbon atom, and "downhill" when bound to an oxygen atom.
Carbohydrates are similar to a fully charged battery, while a water molecule is like a dead battery. But water is a rechargable battery. Plants can recharge it into a carbohydrate. That's the energy-related value of water.

You may have noticed that water tends to cling to itself. It beads up on surfaces and falls from the sky in drops. Insects can even walk on it, if they don't weigh too much.
The tendency of water to cling to itself is called surface tension. It's characteristic of the polar nature of water molecules. The positively charged hydrogen side of a water molecule attracts the negatively charged oxygen side of an adjacent water molecule, forming a daisy chain of hydrogen bonds.
Hydrogen bonds are weak in comparison to covalent and ionic bonds, but they are still important. They not only encourage water to bead up into drops, they also fold proteins into the shapes that make them functional.
