
Garden and Plate
The Molecular Biology of Nutrition











Carbohydrates are versatile molecules. They are created in the green parts of plants, but they don't stay there long. They come in many forms and perform a wide variety of tasks. They start out as simple sugars, but may end up as wood in your desk, starch in your potato, or fiber in your rope. They break down into hydrocarbons, the raw materials for fossil fuels and plastic. If bacteria get to them, they can become alcohol or vinegar. Plants use them as a support material for building rigid structures like branches, and every living thing uses them for transporting and storing biological energy. Very impressive for something that's created out of nothing more than air, water, and sunshine.
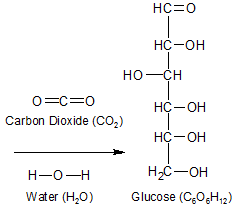
Figure 1 - It takes six molecules each of carbon dioxide and water to make one molecule of glucose. The plant releases the extra oxygen as waste.
Plants use the power of the sun to create carbohydrates out of water and carbon dioxide. The chemical formula for carbon dioxide is CO2, the one for water is H2O, and the one for the single units that make up a carbohydrate is CH2O (see Figure 1). To look at the chemical formulas, you would think the sun's energy would be used to break the bonds in the carbon dioxide molecule, and the liberated carbon atom would attach itself to the water molecule. After all, the resulting molecule is called a carbohydrate, which implies that water was added to carbon (the carbon was hydrated). In nature, unfortunately, things are more complicated than that.
While it's true that plants use energy from the sun to create carbohydrates out of water and carbon dioxide, they do it in a complicated series of chemical reactions called photosynthesis. Photosynthesis transforms the energy contained in sunlight into chemical energy in molecules. The chemical bonds that exist between the oxygen, hydrogen, and carbon atoms in a carbohydrate molecule have higher-energy levels than the chemical bonds in the water and carbon dioxide molecules from which they are derived. These higher-energy chemical bonds are created by re-organizing water and carbon dioxide molecules into a configuration that forces their electrons into higher energy orbits. This higher energy molecular configuration is the carbohydrate.
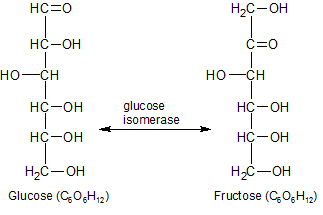
Figure 2 - The enzyme glucose (xylose) isomerase can convert fructose and glucose into each other. This is how they make high fructose corn syrup.
The basic structural subunit of a carbohydrate is CH2O. The carbon atom in CH2O is connected to a hydrogen atom on one side, and an oxygen atom on the other. Carbon atoms like to make a total of four chemical bonds, so they combine with the carbon atoms in the other CH2O subunits, forming a chain with a carbon backbone. You can see how these subunits combine to create glucose and fructose carbohydrates in Figure 2. Because the CH2O subunits automatically combine with other atoms or molecules, they aren't available as individual subunits in the cell. Hydrogen, carbon, and oxygen atoms must be removed from other, more stable molecules in order to build additional CH2O subunits within existing pre-carbohydrate molecules. Photosynthesis gets these additional atoms from water and carbon dioxide, using the energy of the sun to break them down. They are then re-assembled into CH2O subunits within carbohydrates. That's what the "Calvin Cycle" is all about. Once you get the carbohydrates started, they come together in a variety of ways, as shown below.
The smallest biologically significant carbohydrates are called the monosaccharides. Plants get most of their monosaccharides by breaking down the larger carbohydrates (sucrose and starch) that they created in the Post Calvin Cycle reactions. The monosaccharides are similar in many ways, but different enough to exhibit unique chemical behaviors. They all have a minimum of a three carbon backbone, but the most nutritionally significant contain five or six carbons. They all contain high energy bonds.
They can be used for short-term energy storage, or assembled into larger carbohydrates for longer term energy storage. Many of the monosaccharides can also be combined with proteins to form glycoproteins. Glycoproteins provide a wide variety of services, which include signalling between cells, and immune system development.
The molecular structure of each kind of monosaccharide can take different forms, rotating between alpha (α) and beta (β) shapes, or switching between open chain and ring configurations. The chemical differences between these different forms are dependent not only on the differences in the bond connections, but also in the way the atoms are rotated relative to each other. Molecules are three dimensional objects, and their shape affects their function. The two dimensional images below help us to understand the differences, but don't tell the entire story. I've tried to compensate for that in the text. With that in mind, some of the monosaccharides that are important in nutrition are examined below.
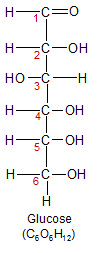
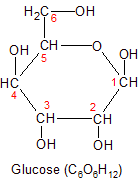
Glucose is the most nutritionally important monosaccharide. It's the only form of carbohydrate that can cross the blood/brain barrier, so it's the only source of energy for the brain. Glucose is also the form in which carbohydrates enter glycolysis, which is the process used by all cells to convert carbohydrate into energy. The other forms of carbohydrate must be broken down or converted into glucose before they can enter glycolysis. Glucose is also the only monosaccharide used in the creation of starch, glycogen and cellulose.
As you can see from the images, glucose has a six-carbon backbone. On the left it's shown in it's open chain form. On the right it's shown in it's ring form. I've placed red numbers next to the carbon atoms to show how they connect in the ring form. Glucose (and other monosaccharides) close up into a ring formation when placed in water. In the case of glucose, carbon-1 and carbon-5 form a ring by bonding to the same oxygen. Note that in both forms, carbon-1 is the only carbon atom that has two of it's four bonds connected directly to oxygen. For this reason it's called the anomeric carbon. The anomeric carbon is the only place where the ring structure can be opened up so the glucose molecule can enter glycolysis and be broken down for energy.
Glucose (and other monosaccharides) can also exist in beta (β) form. This happens when the OH group attached to carbon-1 on the glucose molecule is moved from it's position above the plane of the glucose ring structure, to a position below it. To see how this can cause significant changes in the biological function of a molecule, check out it's role in the cellulose section below.
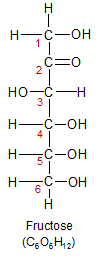
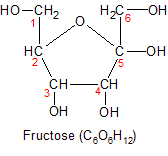
Fructose (another six-carbon molecule) is the second most important monosaccharide, because it's plentiful in fruit (hence it's name), and consumed in large amounts in the form of high fructose corn syrup and table sugar (sucrose). Although it has the same number of carbon, hydrogen and oxygen atoms as glucose, it folds up into a five-sided ring instead of six. This is because fructose has it's carbon/oxygen double bond on carbon-2 instead of carbon-1, making carbon-2 it's anomeric carbon. When it forms a ring, it has two carbons that are excluded from the ring instead of one. This gives it different chemical properties. The most important difference is that it can't be used by most of the cells in the body directly, because it can't be broken down for energy by glycolysis. It must be converted into glycogen or triglycerides by the liver in a process called fructolysis. For more details on the nutritional consequences of the chemical differences between glucose and fructose, see the digestion page.
While glucose and fructose are the monosaccharides that get most of the attention, there are others that are also important for the roles they play. We take a quick look at two of them below.
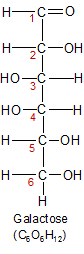
Galactose can be found in a wide variety of foods, but is most often consumed as one of the two monosaccharides in lactose, a disaccharide that's found in milk. Galactose can be combined with proteins or lipids to form glycoproteins or glycolipids, or it can be converted into glucose using a process called the Leloir pathway.
Galactose is a good example of how two molecules that are made of identical atoms can have important functional differences due to small variations in shape. Galactose and glucose are identical except for the placement of the atoms attached to the carbon-4 atom. If you compare the open chain images of the two molecules, you'll see the OH and H groups are on opposite sides of the carbon-4 atom. These are two dimensional representations of three dimensional molecules, so the images don't properly convey the significance of the shape change. The shape change is significant though, because it gives galactose and glucose functionally different characteristics.
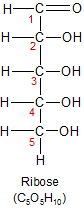
Ribose is a five carbon sugar, so it's different from glucose and galactose not only in it's shape, but in it's atomic composition as well. It's used more as a component of other biological molecules than as a source of energy. It's included in the structures of the ATP and RNA molecules. When it loses the oxygen atom that's attached to carbon-2, ribose becomes deoxyribose. In this form it becomes the base for the nucleotides that link together to create DNA. The loss of the oxygen atom from carbon-2 gives the molecule extra flexability, which makes the double helix shape of the DNA molecule possible.
When you join two monosaccharides together using a glycosidic bond, you get a disaccharide. The mono and disaccharides are collectively referred to as sugars. There are many ways to join monosaccharides together, so there are several different disaccharides. Combining glucose and fructose gives us sucrose, and the galactose-glucose combination gives us lactose. Trehalose and maltose are both glucose-glucose disaccharides, but joined together at different carbons. Trehalose is joined C1 to C1, while maltose is joined C1 to C4. We'll see how the choice of carbons used in the glycosidic bonds affect the chemical properties of the different disaccharides when we compare sucrose to lactose below.
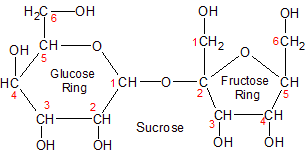
Sucrose is a twelve-carbon disaccharide, composed of fructose and glucose monosaccharides that are connected by a glycosidic bond in a condensation reaction. You are most familiar with it as table sugar. It's one of the two forms of carbohydrate created by plants in the post calvin cycle reactions that follow photosynthesis (starch is the other).
Animals can't absorb disaccharides into the bloodstream, so they hydrolyze sucrose (break it down) into glucose and fructose using the pancreatic enzyme sucrase in the small intestine. Animals (including us) then absorb it into the portal vein as fructose and glucose, and have no reason to re-assemble it back into sucrose. The fructose and glucose enter the liver, where they go their separate ways.
Sucrose is much more important for plants because it's the form in which they transport carbohydrate throughout their systems. Some plants, like sugar cane and sugar beets, also use it for storage instead of starch. Sucrose is the perfect choice for transport because it's a non-reducing sugar, which makes it more biologically stable.
What does it mean to be a non-reducing sugar? It means that the carbon-oxygen bond of the anomeric carbon in a sugar's ring isn't exposed. If it isn't exposed, the bond can't be broken down (reduced), converting the ring into an open chain form.
In most disaccharides, the glycosidic bond that holds the two monosaccharides together use the anomeric carbon of one monosaccharide, but not the other. For example, lactose uses the anomeric carbon of galactose (carbon-1) in it's glycosidic bond, but not glucose's anomeric carbon (carbon-1). This leaves the glucose ring in lactose exposed to reduction. Once the glucose ring is reduced, the galactose ring becomes exposed and is also reduced.
Compare this to sucrose, which is a non-reducing disaccharide. The glycosidic bond in sucrose uses the anomeric carbons of both glucose (carbon-1) and fructose (carbon-2), leaving no anomeric carbon exposed. This is what it means to be a non-reducing sugar. The glycosidic bonds can be broken in sucrose, but it takes sucrase in animal intestines, or sucrose synthase or invertase in plants to get the job done. Sucrose and trehalose are the only two non-reducing disaccharides.
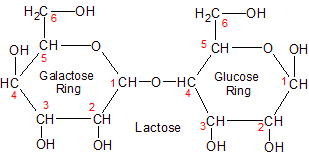
Another disaccharide is lactose, a combination of glucose and galactose. Plants contain galactose, but they don't combine it with glucose. They prefer the disaccharide sucrose, because they need something that is durable enough to be transported throughout the plant without being reduced along the way.
Mammals have different needs than plants, though, so they create lactose for their young. Lactose is included in the milk of mammals because it has qualities that make it especially useful for the metabolism of infants. Lactose is more easily digested (reduced) than sucrose, and the galactose it contains is used for cell signaling and immune system development, both of which are essential for infants. Galactose is far more useful than the fructose that's found in sucrose, making it the preferred monosaccharide for infant mammals.
Lactose is separated into galactose and glucose by the lactase enzyme in the small intestine. Humans produce lots of lactase when they're infants, but some people stop producing it as they get older, making them lactose intolerant. The lactose proceeds undigested into the large intestine where bacteria are happy to digest it. The large intestine isn't where you want digestion to occur, as it creates the symptons associated with lactose intolerance (gas pains and diarrhea due to the production of carbon dioxide).
The largest carbohydrates of all are the polysaccharides. They consist of large (200 to 2500 carbon backbone) molecular structures made from combining many glucose monosaccharides. There are three kinds of polysaccharides:
|
|---|
While all three of these polysaccharides are assembled from large numbers of glucose molecules, they are combined in three different ways. Starch is composed of two different polysaccharides (amylopectin and amylose), both of which are strings of α-glucose molecules, combined into two different (branched and unbranched) structures. Glycogen is similar to amylopectin, but more highly branched. Cellulose is unbranched like amylose, but uses β-glucose molecules instead.
While sugar cane and sugar beets prefer to store their carbohydrate as sucrose, most plants use starch for long term storage. Seeds contain starch while they lie dormant, then convert it into glucose as the seed germinates. Corn and wheat are full of starch because they are seeds. Potatoes are full of starch because they are the storage centers for the potato plant. Fruit trees and berry bushes fill their fruit and berries with starch until it's time for them to ripen, then they convert the starch into glucose, fructose, and sucrose as part of the ripening process.

Starch is a combination of two structurally different polysaccharides, called amylose and amylopectin. Amylose is a single linear strand, while amylopectin is a branched version. They are both made by linking glucose molecules together in long chains. In amylose, the glucosyl units are all connected by 1,4 glycosidic bonds in a long, linear chain. The Carbon-1 atom on one glucose is bound to the carbon-4 carbon atom on another glucose molecule through bonds with a shared oxygen atom.
Amylopectin uses the same 1,4 glycosidic bonds that amylose uses, but branches off from the linear 1,4 chain about once every thirty glycosyl units. It uses a branching enzyme to form a 1,6 glycosidic bond between two strings. This leaves the carbon-4 atoms on the glucosyl units at the ends of both strings free to create 1,4 bonds, producing a branch.
Starch is composed of approximatley 25% amylose, and 75% amylopectin, depending on the plant. Because enzymes can only break down starch from it's ends, Amylopectin is easier to breakdown, because it's branched structure has more end points than amylose. There's a detailed explanation of how plants create starch on the Post Calvin Cycle page, and another one that describes how plants and animals break starch down on the Digestion page.
Animals create fat when they need long term energy storage, and use glucose when they need energy right away. Glycogen is more of an intermediate energy source for animals, and isn't used by plants at all. Most glycogen is created in the muscles and the liver where it's assembled from the same glucosyl units that are used by plants. Glycogen branches off even more often than the amylopectin that's used in plants (once every ten glycosyl units instead of every thirty), and is therefore more compact.
It's no accident that most glycogen is produced and stored in the liver and muscles, since it needs to be available whenever glucose levels go down. Glucose levels fall rapidly in muscles during physical activity, which is why glycogen is on standby there. Glycogen is stored in the liver because that organ is the gateway through which glucose enters the bloodstream from the intestine. Liver cells (hepatocytes) are able to either create or break down glycogen, thereby maintaining glucose levels in the bloodstream. See the Digestion page for more details.
Glycogen, like starch, is made up of glycosyl units that are derived from glucose and interconnected by glycosidic bonds in a dehydration reaction. It's connections use the same carbons as starch, making carbon-1 to carbon-4 bonds for the linear strings, and carbon-1 to carbon-6 bonds for the branches. There are, however, some differences. While starch is stored inside plastids (chloroplast and amyloplast) in plant cells, Glycogen is stored within the cytosol of the cell in animals.

Cellulose is similar to amylose (starch) because they are both linear strands of glucosyl units. Neither molecule forms branching structures using 1,6 carbon bonds. They both use 1,4 bonds exclusively. Cellulose is different from amylose in that it's made out of β-glucose while amylose is made from α-glucose. Small changes in shape can make big differences in biological function.
α and β glucose have identical bonds which differ only in the positioning of the carbon-1 hydroxyl (OH) group relative to the rest of the glucose molecule. α-glucose has the OH group positioned above the plane of the glucose ring, while β-glucose has the OH group positioned below the plane of the glucose ring. The β-glucose is still connected by 1,4 glycosidic bonds, but because of the change in OH position, the amylase enzymes that are used to sever the α-glucose bonds in starch can't break the β-glucose bonds in cellulose. A suite of cellulase enzymes is used instead. Plants and animals don't have these enzymes, making cellulose indigestible for all organisms except a few bacteria, fungi and protozoans. A certain class of mammals called ruminants contain cellulose-digesting bacteria in their guts. That's why animals like cattle and goats can consume grasses like hay. Termites are able to eat wood because they also contain cellulose-digesting bacteria in their guts.
Because cellulose doesn't branch or coil at all, individual strands of it are able to snuggle up next to each other in a tightly packed configuration that's very strong. The hydroxyl (OH) groups on one strand of cellulose form hydrogen bonds with the OH groups on adjacent strands, forming cellulose structures that are tough and rigid. Plants use cellulose as a structural material in their cell membranes. Wood is rigid because it's made primarily from cellulose. It's hard to believe, but even the dry parts of the Chinese Elm tree trunk shown above are almost exclusively made from carbon dioxide and water that's been re-arranged into cellulose. The widespread use of cellulose in the plant kingdom makes it the most abundant organic compound on the planet.
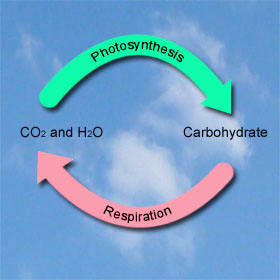
Photosynthesis is a set of chemical reactions that use the energy of the sun to re-arrange the chemical bonds of water (H2O) and carbon dioxide (CO2) into the higher-energy C-C and C-H bonds in carbohydrates).
Respiration reverses this process, using the stored energy for biological functions. It breaks catbohydrates down into water and carbon dioxide, which completes the cycle.
If the short explanation isn't enough, you can find additional information on carbohydrates at the following pages:
Post Calvin Cycle reactions pick up where photosynthesis leaves off. They show you how plants create starch and sucrose from the products of photosynthesis.
Digestion is all about how carbohydrates get from their hiding places in fruits, grains and vegetables into the cells of animals.
Fructolysis is the process in which the body converts fructose into energy. Fructose can't take part in glycolysis (the first stage of respiration), so it goes through fructolysis in the liver instead.
Creating carbohydrates is all about storing energy. Sounds simple enough. But when you look at photosynthesis and respiration, things get very complicated. Energy is added in one step just to be released in another. Molecules are split apart and re-assembled later for no apparent reason. Six molecules are created, but only one can be used. Phosphate groups are added and removed everywhere. There's a whole lot of shuffling going on. What's the bottom line?
The bottom line is this: The "low-energy" bonds in water (H-O-H) and carbon dioxide (O=C=O) are being re-arranged into the "high energy" (C-C and C-H) bonds in glucose. If you compare the molecular structure of water and carbon dioxide to that of glucose, you'll see that water and carbon dioxide have no C-H or C-C bonds, while glucose has seven C-H bonds and five C-C bonds (see figure 1 image). Photosynthesis rearranges O-H and O-C bonds into C-H and C-C bonds. That's what it's all about.
What's so special about C-H and C-C bonds? That's where things get complicated again. The simple answer is that the outer (valence) electrons in hydrogen and carbon are in a higher position (potential energy state) when they are shared in a covalent bond with carbon than when they are shared in a covalent bond with oxygen. That's because oxygen has a much stronger pull (electronegativity) than carbon, so carbon's shared electrons are gripped more tightly by oxygen in a C-O bond than they are by the opposite carbon in a C-C bond. It's the transfer of the shared electron from a C-C or C-H bond to a C-O or H-O bond that releases the energy, not the bond itself.
It's a little misleading to call C-C and C-H bonds "high energy". It's like saying that a boulder sitting fifty feet up the side of a hill has more energy than one on the valley floor. They are both just sitting there, but the fifty-foot high boulder has the potential to release energy if given a small push downhill. The boulder at the bottom of the hill doesn't.
Cellular respiration uses enzymes to provide the small push that ultimately results in the "downhill" conversion of C-C and C-H bonds into C-O and H-O bonds, with the corresponding release of chemical energy. Photosynthesis uses the energy of the sun to push the electrons "uphill" by rearranging the C-O and H-O bonds in carbon dioxide and water back into the C-C and C-H bonds in the carbohydrates.
The storage and release of energy isn't confined only to rearrangements of carbon, hydrogen and oxygen bonds. Other bonds are used as well. ATP uses P-O bonds to accomplish the same thing, while NADPH and NADH transfer electrons rather than entire atoms. Other factors, such as the electrostatic repulsion created by forcing electrons close together (also used by ATP) is another way to "store" energy. If you want to delve deeper into how energy is stored and released in biological molecules, see the other pages on Energy, photosynthesis, and cellular respiration.
ATP and NADPH store their energy in the chemical bonds between phosphate groups (ATP) or in high energy electrons (NADH and NADPH). This is a short-term, readily-available form of energy that can quickly be used for many biological processes, including the creation of carbohydrates.
If ATP and NADPH can do this, why do we need carbohydrates? Here's a few reasons:
Each molecule has it's role, and all of them are necessary.
Fiber is just another word for cellulose, the carbohydrate that we can't digest. We tend to use cellulose when we're talking about it's structural characteristics in plants, and fiber when we talk about it's nutritional value in humans. They are the same thing, referred to in different contexts.
How can something that's indigestible have nutritional value? It has value as bulk in the digestive process. While the proteins and starches are broken down and absorbed into the bloodstream, the fiber stays behind. It also dilutes the other nutrients so they can be digested and absorbed in a slower manner.
Why is slower digestion better? Because for millions of years we evolved by eating plants that were high in fiber. Slow digestion isn't inherently better, it just happens to be the way our digestive system adapted to an environment filled with high fiber foods. Our guts have trouble adjusting to a fiber-free diet, so we should keep eating high fiber foods. This way we can hopefully avoid the consequences of a low-fiber diet. Things like irritable bowel syndrome and colon cancer.
When looking to add high fiber foods to your diet, remember that plants create fiber (cellulose) and animals don't. So foods like meat, dairy and eggs don't have any fiber, while fruit, grains, and vegetables do. Highly processed foods like sugar and white flour have had all of the fiber removed, so look for foods like whole wheat and brown rice.

Celery is often used as the poster child for fiber content in food because of the many strings of cellulose that get caught in your teeth while eating it. Celery is an example of a food that's high in carbohydrates yet low in calories, because the carbohydrates are in a form (fiber) that can't be digested. It's too bad fiber isn't sweet like fructose. It would be the ultimate non-calorie sweetener.

Alcohol is created when sugar can't break all the way down into carbon dioxide and water due to a lack of oxygen. There are several different alcohol molecules, but the one that's in our alcoholic beverages is called ethanol.
Ethanol is produced from the acetaldehyde molecule that normally enters the citric acid cycle as part of the acetyl CoA molecule. A lack of oxygen blocks this process, so the alcohol dehydrogenase enzyme converts the acetaldehyde to ethanol instead. Converting sugar into ethanol is called fermentation. When we give sugar to yeast in an oxygen-free environment, they produce ethanol.
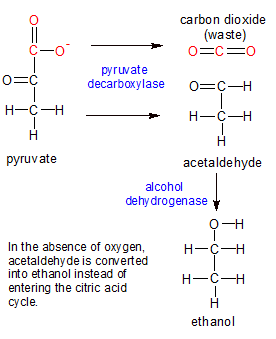
Alcohol has unique qualities because it's been metabolically suspended between two types of molecules. It still has the CH bonds of a sugar on one end, but now has the OH bond of a fatty acid molecule on the other. Cell membranes are constructed out of fatty acids, so the small ethanol molecule is able to penetrate the cell membrane. This disrupts it's ability to function properly. If ethanol blood levels get too high, death can occur.
Alcohol dehydrogenase can convert ethanol back into acetaldehyde if oxygen becomes available, but that process takes time. When we consume too much alcohol at once, our ability to convert it back into acetaldehyde is overwhelmed. The excess ethanol circulates in the bloodstream. It's a small molecule, so it's able to cross the blood/brain barrier. When this happens, it interferes with the ability of the brain to function properly. This causes the symptoms known as inebriation.
The same carbohydrates that power our bodies are also the raw materials from which oil, coal and gas originate. Hydrocarbons (the fossil fuels), are created when all of the oxygen is removed from the carbohydrates (usually due to the high heat and pressures of geological processes). This is how natural gas, coal deposits and oil fields came into existence.

It has been suggested that carbohydrates were able to decompose into fossil fuels in the distant past because of the lack of oxygen (and aerobic organisms) in the atmo-sphere. Today there are plenty of aerobic organisms that are able to break carbo-hydrates all the way down into water and carbon dioxide, so the fossil fuels are no longer being created.
If you apply additional geological heat and pressure to hydrocarbons, you'll remove the hydrogen and end up with pure carbon. If you squeeze the pure carbon even more, you eventually get diamonds.
Carbohydrates aren't always found working solo. They fill many roles. When they aren't busy providing structural support or doing their energy thing, they team up with fats and proteins to help out with a wide variety of other functions. In most cases, short strings of three to ten monosaccharides collectively called the oligosaccharides are the ones to pair up with the fats and proteins.
In addition to all of the other names that fill the carbohydrate universe, you may have heard people talk about the simple and the complex carbohydrates. These terms divide all carbohydrates into two main categories. Simple carbohydrates include the mono and disaccharides while complex carbohydrates include anything over two monosaccharides in length. This includes the oligosaccharides and polysaccharides.

Now that we know the structural differences between the various carbohydrates and how the body uses them, we can ask meaningful questions about how they provide nutritional value that benefits our health. The Plate page has some practical tips on how to use this information at mealtime.