
Garden and Plate
The Molecular Biology of Nutrition










There are nine water soluble vitamins, eight of which are members of the vitamin B complex. The remaining water soluble vitamin is vitamin C. While vitamin C and the four fat-soluble vitamins (A, D, E and K) are composed entirely of hydrogen, carbon and oxygen atoms, all of the B vitamins contain at least one nitrogen atom. Two of the B vitamins (thiamin and biotin) also contain a sulphur atom, and vitamin B12 (Cobalamin) contains a cobalt atom.
The large number of oxygen and nitrogen atoms make these vitamins water soluble because the relatively high electronegativity of these atoms compared to carbon and hydrogen make the molecules polar. Since water molecules are also polar, water-soluble vitamins move through (and out of) the body easily. This makes it difficult to reach toxic levels, but easy to have deficiencies, so these vitamins are added to several fortified foods. We look at these vitamins below.
 |
Vitamin B1 (thiamin) can be found in virtually all unprocessed foods because it's involved in biological processes that are common to all life. It's found in the highest concentrations in whole grains, yeast, nuts, sunflower seeds, pork and liver. In the grains it's found mostly in the outer layer, which is why wheat bran is especially high in thiamin and polished rice is especially low. In many parts of the world thiamin is added back into processed foods like white rice to compensate for losses due to processing. Thiamin is produced in fungi, bacteria and plants. Animals must get it from the foods we eat because we lack the enzymes needed to synthesize the thiamin molecule ourselves. A deficiency of thiamin in the diet results in a disease called Beriberi.
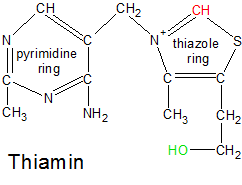
Thiamin becomes active when two phosphate groups are attached to its green oxygen atom, converting it into thiamin diphosphate (TDP). These groups allow it to combine with transketolase, which positions thiamin's red carbon near a target molecule where it's able to sever or create C-C single bonds that exist adjacent to C=O double bonds. |
Thiamin is composed of two connected ring structures, with nitrogen, oxygen and carbon atoms hanging off of the rings. The OH tail attached to one end of the thiamin molecule is important because phosphate groups make the thiamin molecule biologically active when they are bound to it. There are three phosphorylated forms of Thiamin. If one phosphate group is attached to the oxygen atom on Thiamin's tail, it's called thiamin monophosphate (TMP). If it has two phosphate groups, it's called either thiamin diphosphate (TDP) or thiamin pyrophosphate (TPP). If it has three phosphate groups it's called thiamin triphosphate (TTP). Thiamin monophosphate has no known biological function, while thiamin triphosphate's role is still being investigated. Thiamin diphosphate, on the other hand, is an essential participant in multiple metabolic processes. We'll look at thiamin first, and then TDP.
When created in plants, thiamin is assembled as thiamin monophosphate (TMP) from two precursor molecules. Each molecule contains one of the ring structures. The molecule that contains the ring with the sulphur atom is called the thiazole ring, and has one phosphate group attached to it. The molecule that doesn't contain sulphur is called the pyrimidine ring, and is bound to two phosphate groups. TMP is formed when the ring structures are bound together and two of the three phosphate groups are discarded. This synthesis occurs inside the chloroplasts of plant cells.
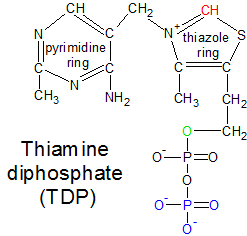
TDP with its two phosphate groups, one of which I have highlighted in blue. This is the biologically active form of thiamin. |
TMP isn't useful in its current form, and must be converted into TDP in order to become biologically active. TMP is first stripped of its single phosphate group to become thiamin. Thiamin is then converted into TDP by the enzyme thiamin diphosphokinase, which transfers two phosphate groups from ATP to thiamin. This results in TDP and AMP. The AMP is discarded, and the biologically active TDP molecule is used by several different enzymes. The conversion of thiamin into TDP is easily reversible and biologically useful because thiamin is more mobile than TDP. The attached phosphate groups make it difficult for TDP to cross cell membranes.
While some of the enzymes that create thiamin are missing in animals, the enzymes that use TDP are common to all organisms. This is because TDP is essential for photosynthesis and respiration. The pyruvate dehydrogenase enzyme uses TDP to convert pyruvate into acetyl CoA in the second stage of respiration, and the transketolase enzyme uses TDP in steps nine and twelve of the Calvin Cycle to transfer a ketone group from one molecule to another. A ketone group is part of a molecule that contains a carbon and oxygen double bond (C=O). The double-bonded pair of atoms by itself is called a carbonyl group. Molecules that have carbonyl groups on their ends are called aldoses, while molecules that have carbonyl groups inside a molecule's carbon chain are called ketoses. The enzyme transketolase uses TDP to transfer a ketone group from a ketose to an aldose. Transketolase can't transfer the ketone group unless TDP is attached to it. Let's see how this reaction works.
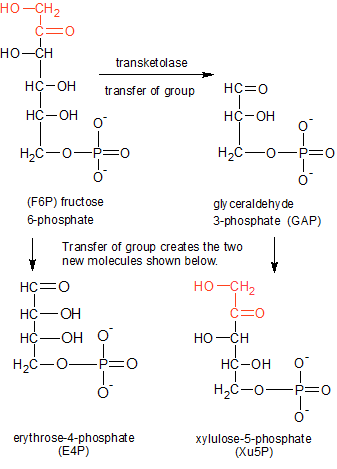
Transfer of the red two-carbon ketone group (C2H3O2) from F6P to GAP creates E4P and Xu5P. The transketolase enzyme uses TDP (not shown) to make the transfer. |
In step nine of the Calvin Cycle, the transketolase enzyme uses the TDP molecule to transfer a ketone group from fructose 6-phosphate (F6P) to glyceraldehyde 3-phosphate (GAP). This will convert the F6P ketose into an aldose called erythrose 4-phosphate (E4P), and the glyceraldehyde 3-phosphate (GAP) aldose into a ketose called xylulose 5-phosphate (Xu5P). For this to take place, the molecules must be positioned correctly. First, TDP buries itself in a channel between the two identical halves of transketolase, with only the red CH group on the thiazole ring sticking out (see the TDP diagram). The oxygen atoms in TDP's phosphate groups form hydrogen bonds with the inside of the enzyme, anchoring TDP in place. This is why thiamin is only biologically active after it becomes TDP through the addition of two phosphate groups. The combination of transketolase and TDP form an active site where the substrate molecules are positioned and altered.
The active site on the enzyme attracts a molecule of F6P and holds it in place with hydrogen bonds. The enzyme positions TDP and F6P close to each other and the reaction begins. The hydrogen atom attached to the red carbon on TDP's thiazole ring has a weak grasp on the electron pair it shares with the carbon atom due to the influence of the positively charged nitrogen atom next to the red carbon. When F6P is positioned close to the thiazole ring, the hydrogen atom's nucleus (a proton) is forced to break its bond with the red carbon and leaves its electron behind. This leaves the red carbon (on the thiazole ring) with an unused electron pair, making it a carbanion.
The carbanion uses its negative charge to sever the bond between carbons 2 and 3 (from the top) in the F6P carbon chain. This bond is easier to sever than other C-C bonds because one of the carbons (C2) is also involved in a double-bond with oxygen. The C=O (carbonyl) double bond weakens the adjacent C-C single bond that the carbanion targets. The carbanion then binds to the severed two-carbon ketone group (which also contains two oxygen and some hydrogen atoms), while the rest of the F6P molecule becomes E4P.
E4P diffuses away, and GAP takes its place. The carbanion on TDP's ring is exposed to the carbonyl group on the end of the GAP aldose, and the ketone group is transferred over to the GAP molecule, where its C2 atom forms a bond with C1 on the top end of the GAP molecule. This converts the GAP aldose into the Xu5P ketose, and the reaction is complete. It should be noted that transketolase can run this reaction in reverse because the molecule that gained the ketone group (GAP to Xu5P) is now a ketose molecule, and can just as easily lose its newly acquired ketone group in an identical reaction. Within the larger context of the Calvin Cycle, though, the two products of this reaction will move on to other enzymes and complete the Calvin Cycle.
Riboflavin can be produced by bacteria, fungi, plants and some animals. Humans and other mammals can't synthesize it, so we must get it from our diet. Riboflavin is found in most unprocessed foods, and is especially high in yeast and some organ meats like the liver and kidneys. Grains contain moderate amounts in their outer coats, but lose most of that when they are processed. Riboflavin is added back into some processed foods, but not white rice due to riboflavin's yellow color.
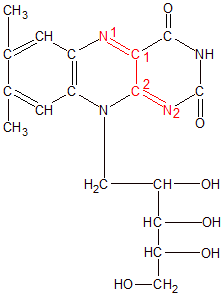
Riboflavin is shown here in an oxidized state. Its active site is in red. It must become part of FMN or FAD before it can participate as a cofactor with an enzyme in a chemical reaction. |
Riboflavin is composed of a three-ring structure called an isoalloxazine, with a ribose-like side chain called ribitol attached to its center ring. As with thiamin, riboflavin isn't biologically active until more components are attached. The addition of a single phosphate group to the far end of the ribitol side chain produces a biologically active molecule called flavin mononucleotide (FMN). When a molecule of adenosine monophosphate binds its phosphate group to the phosphate group on FNM, we get a molecule called flavin adenine dinucleotide (FAD). In both molecules it's the isoalloxazine ring structure that performs the reaction. The phosphate groups and other components are used to attach the molecule to the enzyme and position it near the molecules that need to be oxidized or reduced. FAD is more biologically active than FMN because it's used by more enzymes.
FADH2 is the reduced form of FAD. Remove the blue section to get FMNH2. Riboflavin is in red and black. |
FMN and FAD attach to and work with nearly one hundred different enzymes, but always function in the same manner. They acquire extra electrons by forming covalent bonds with one or two hydrogen atoms, then donate the extra electrons to other molecules as needed. When they form a bond with only one hydrogen atom they are referred to as FMNH and FADH. When they form bonds with two hydrogen atoms they become FMNH2 and FADH2. They have this flexability because of the way the nitrogen and carbon atoms are placed within the three-ring structure.
As you can see in the Riboflavin image, N1 has a double bond with C1 and N2 has a double bond with C2. C1 and C2 have a single bond between them. When hydrogen atoms bond to N1 and N2 as shown in the FADH2 image, the N=C double bonds become single bonds, and the C1-C2 single bond becomes a double bond.
The bond changes allow the FMNH2 or FADH2 molecule to hold onto the extra hydrogen atoms in a stable configuration until the electrons are needed elsewhere. When the electrons are donated during chemical reactions, the bonds revert to their former configuration. These are versatile molecules because they can accept and/or donate both hydrogen atoms at once, or one at a time, and they can assemble the atoms from any combination of protons, electrons or hydride ions (a proton with two electrons) from the surrounding water or other molecules.
Riboflavin (in the form of FAD and FADH2) plays a central role in respiration. The pyruvate dehydrogenase complex uses it to transfer two electrons to NAD+, creating a molecule of NADH during the second stage of respiration known as pyruvate decarboxylation. Additional kinds of dehydrogenase enzymes also use FADH2 to transfer electrons from other molecules to NAD+ at multiple steps of the Citric Acid Cycle to produce more NADH. These reactions are important because NADH will go on to transfer the electrons to the electron tranport chain where they'll provide the energy needed to create ATP and complete the process of respiration.
|
|
Niacin is a small molecule composed of a single pyridine ring with a functional group attached. It comes in two varieties, nicotinic acid and nicotinamide. The only structural difference between the two is in the functional group. Nicotinic acid has a carboxyl (COOH) group, while nicotinamide contains a carboxamide (CONH2) group. The small structural difference produces large functional differences between the two niacin molecules because the differences determine which enzymes and other molecules each type of niacin is able to work with. The functional groups of each type of niacin are colored red in the adjacent diagrams.
Plants and some microorganisms create niacin's pyridine ring from dihydroxyacetone phosphate and aspartate, but animals have to acquire both forms of niacin from the food they eat. Niacin is found in almost all unprocessed foods, and is added back into some foods where excessive processing has removed it. Plants contain significant amounts of both forms of niacin, but in some cases most of their nicotinic acid is unusable because it's chemically bound to sugar molecules which form undigestable molecules called glycosides.

Chicken and rice both provide niacin. Chicken contains niacin as part of the energy carrier molecule NAD. It also provides tryptophan, which can be converted into niacin. The outer shell of whole grain rice contains niacin, but white rice loses its niacin when it loses its shell. White rice can still be a good source of niacin if it is added back in during processing, which is often the case. |
Most animal-based foods contain relatively small amounts of nicotinic acid because the liver converts most of what isn't needed into nicotinamide. These foods are high in protein, though, which contain the essential amino acid tryptophan. This means we can use meat as an alternative source of nicotinic acid when necessary. We can produce one mg of nicotinic acid from sixty mg of tryptophan. Tryptophan doesn't free us from our dietary dependence on obtaining nicotinic acid because it also comes exclusively from the foods we eat. Our inability to ingest and/or store large amounts of nicotinic acid leaves us vulnerable to niacin deficiencies which manifest themselves as the condition known as Pellagra.
Nicotinamide is easier to acquire. Both plants and animals contain significant amounts of nicotinamide because it's the active component of nicotinamide adenine dinucleotide (NAD), which is a molecule that most life uses for respiration. NAD is found in the cells of most living things, so it's where we get the majority of our nicotinamide.
We absorb both forms of niacin through a mixture of passive and assisted diffusion. This occurs primarily in the intestine, but also in the stomach. If nicotinamide is ingested as part of a NAD molecule it must be separated out before it can be absorbed. Meats like tuna and chicken are great sources of niacin because in addition to providing nicotinamide from NAD they can also produce nicotinic acid from tryptophan. Liver is an even better source, since it has the added advantage of being the place where niacin is processed and stored for future use. Fortified cereals, peanuts and mushrooms are great sources for biologically available nicotinic acid. Raw foods are higher in niacin than their cooked counterparts because significant amounts of niacin are lost during cooking.
Since niacin is a water soluble vitamin, neither form stays very long in the bloodstream. Red blood cells absorb some of it, but the rest circulates to the liver where it's processed. Some of what reaches the liver is combined with ADP ribose back into NAD, while excess amounts are combined with other molecules and eliminated in the urine.
Nicotinic acid can be converted into nicotinamide when it's incorporated into NAD, or it can go off and do its own thing. Due to nicotinic acid's possession of a carboxyl (COOH) group, it can perform biological activities that are impossible for nicotinamide. One such activity is its ability to reduce cholesterol levels in the blood.
Nicotinic acid accomplishes this by attaching its carboxyl group to a protein called Niacin Receptor 1 that's embedded in the membranes of fat cells. This triggers a change in the shape of niacin receptor 1 inside the cell, which starts a chain-reaction of events that inhibit the breakdown of triglycerides into fatty acids and glycerol inside the fat cells. This in turn leads to lower lipid and cholesterol levels in the blood. For this reason nicotinic acid supplements are sometimes given to patients who have high cholesterol. Nicotinamide can't be used in this way because it doesn't have a carboxyl group.
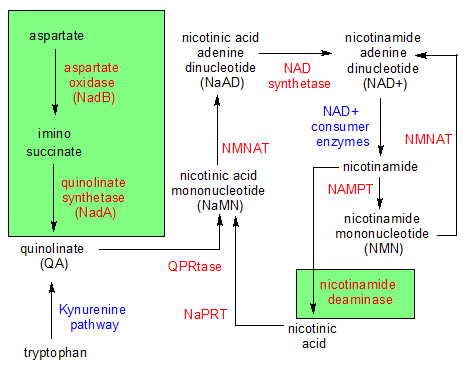
The niacin family of molecules. The molecules are black while the enzymes that change the molecules are red. The blue items are multiple enzymes or entire pathways and the green areas contain reactions that animals can't perform. |
Although nicotinic acid and nicotinamide have their differences, they can both be used in the creation of an important molecule called nicotinamide adenine dinucleotide (NAD). Plants begin creating niacin's ring structure when the aspartate oxidase enzyme converts aspartate into imino succinate. The ring structure is completed in a second reaction when quinolinate synthetase combines part of a dihydroxyacetone phosphate (DHAP) molecule with imino succinate to form a molecule of quinolinate (QA).
QA is where niacin's ring structure is formed, and only plants (and some micro-organisms) can produce it. QA is the same as nicotinic acid, except it has an extra carboxyl (COOH) group. Once the ring structure is synthesized in QA, both plants and animals can participate in the remaining reactions that lead to the creation of NAD+.
The enzyme quinolate acid phosphoribosyltransferase (QPRtase) combines QA with ribose 5-phosphate to create a molecule of nicotinic acid mononucleotide (NaMN). Two important things take place during this reaction. First, the nitrogen atom on QA forms a bond with a carbon on ribose 5-phosphate's ring structure. This forces the nitrogen atom into a net positive charge because it has to manage four covalent bonds when it's normally capable of only three bonds. This is what enables it to become an electron carrier. In the process, a second thing happens. QA loses one of its carboxyl groups, turning it into nicotinic acid.
In the next reaction, NaMN is converted into nicotinic acid adenine dinucleotide (NaAD) through the addition of a molecule of AMP to NaMN's phosphate group by the nicotinamide mononucleotide adenylyltransferase (NMNAT) enzyme. This has the effect of converting the non nicotinic acid part of the molecule from ribose 5-phosphate to ADPribose.
In a final reaction, the NAD Synthetase enzyme converts NaAD into a molecule of NAD+ by converting the nicotinic acid part of the molecule into nicotinamide. This is accomplished by replacing the COOH functional group with CONH2. This completes the synthesis of NAD+ from scratch using a biological pathway within the cells of plants.
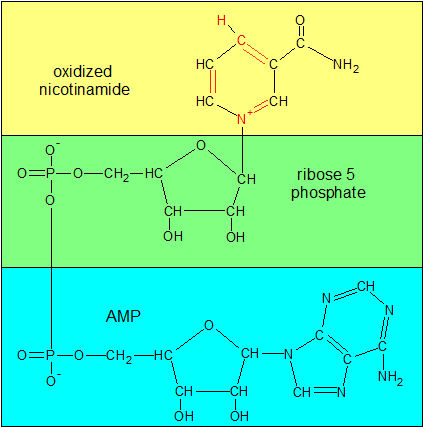
NAD+ is one of the oxidized forms of NAD. The other is NADP+. In this state it's ready to receive two electrons, one of which arrives as a hydrogen atom. To see what it looks like with the extra electrons, see the NADHP image. I have color-coded the sections that get combined at each stage in the formation of NAD+. The green and blue sections taken together form ADP ribose. |
In addition to the comprehensive biological pathway we just examined for the creation of nicotinic acid, nicotinamide and NAD+, there are additional salvage pathways that animals can use to produce free niacin and NAD+ as needed. In one such example, a group of enzymes collectively referred to as NAD+ Consumers hydrolyze NAD+ into ADPribose and free nicotinamide. This might be done in the intestine in order to absorb nicotinamide, or in other parts of the body as a byproduct of other reactions. Free nicotinamide can also be re-assembled back into NAD+ in a two-step pathway that starts with enzyme nicotinamide phosphoribosyltransferase (NAMPT) binding nicotinamide to ribose 5-phosphate, creating nicotinamide mononucleotide (NMN), and ends with NMNAT attaching a molecule of AMP to NMN to produce NAD+. Those are the salvage pathways for the creation and absorption of free nicotinamide.
As for nicotinic acid, things get a little more complicated. Plants and micro-organisms can use the nicotinamide deamidase enzyme to convert nicotinamide into nicotinic acid, but animals can't do this. Fortunately, micro-organisms that live in our intestine convert it for us. What we can do by ourselves is convert an amino acid called tryptophan into QA. This is a multi-step process called the Kynurenine pathway. The QA then becomes NaMN, which contains nicotinic acid. It's a very inefficient process, creating only 1mg of nicotinic acid for every 60mg of tryptophan. This process is even more limited by the fact that we can't create tryptophan, and must get it from our diet. Meat is our best source of tryptophan.
If we have extra nicotinic acid and want to create more NAD+, we can do that ourselves. While nicotinic acid can't be directly converted into free nicotinamide, it can be converted indirectly during a process that incor-porates nicotinic acid into a molecule of NAD+. The enzyme nicotinic acid phosphoribosyl transferase (NaPRT) begins the process by combining nicotinic acid with ribose 5-phosphate. This creates a molecule of nicotinic acid mononucleotide (NaMN). The rest of the process is identical to the last two steps in our earlier example: NaMN is converted into NaAD by NMNAT, then NaAD is converted into NAD+ by NAD synthetase.
The production of NAD+ is an important role in which both forms of niacin participate. Now that we've seen how both nicotinic acid and nicotinamide are converted into NAD+, we'll examine the different forms of NAD and how niacin is crucial for their ability to function.
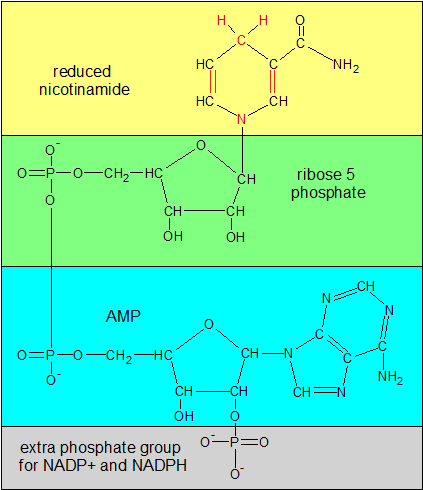
NADPH is one of the reduced forms of NAD. Compare the red parts of nicotinamide in this image with the same area in the NAD+ image. In this image N has lost its positive (+) charge and there's an extra H atom on top of the ring. The bonds on the ring have also shifted position. If you remove the gray phosphate group on the bottom, you'll have NADH, the other reduced form of NAD. |
You may have noticed in the earlier sections that I started talking about NAD, and then switched over to NAD+. That wasn't an accident. NAD refers collectively to a group of four similar molecules, while NAD+ is the version of NAD that's originally created when niacin and the other NAD components are combined. NAD+ and NADH are the oxidized and reduced forms of one pair of molecules, while NADP+ and NADPH are the oxidized and reduced forms of those molecules with an additional phosphate group attached.
The active site in NAD is the niacin ring structure, but it isn't activated until it becomes part of the NAD+ or NADP+ molecule. The + in NAD+ refers to the positively charged nitrogen atom in niacin's ring structure. The nitrogen atom acquires this positive charge when it's forced to bond with a carbon atom during the formation of NaMN (A precursor of NAD+). Nitrogen is stable when it forms three bonds, but is forced in NaMN to create a forth bond. This is what gives it a positive charge and enables the ring to pick up two electrons. When NAD+ and NADP+ are reduced to NADH and NADPH2, an electron is added to the nitrogen atom, removing its positive charge. An extra hydrogen atom is also added to the red carbon atom on the opposite side of the ring (see the image). These additions force the single and double bonds in the ring structure to shift states, just as it does in riboflavin's ring structure.
The ring structure works the same way in NADP+ as it does in NAD+, but the extra phosphate group in NADP+ causes it to participate in different biological reactions than NAD+. While each pair of molecules easily fluctuates between its oxidized and reduced state during reactions, the extra phosphate group makes each pair a unique tool to be used by a different set of enzymes for different tasks. While NAD+ participates mostly in energy production through the creation of ATP by donating its electrons to the electron transport chain, NADP+ uses its electrons to help synthesize other types of molecules like cholesterol and fatty acids. In the next section we'll see how riboflavin (as part of FAD) transfers two electrons to niacin (as part of NAD+) during the breakdown of pyruvate into acetyl CoA.
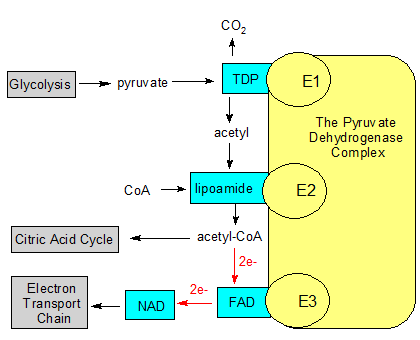
The yellow pyruvate dehydrogenase complex is made up of multiple copies of three different enzymes. The substrates (the black text without boxes) are modified using molecular tools (in the blue boxes). The gray boxes represent processes that produce or use the substrates. The red arrows with 2e- show how FAD accepts two electrons and then passes them on to NAD. |
Now that we've looked at the first three B vitamins, let's see how they work together in a process called pyruvate decarboxylation, or the second stage of respiration. It accepts pyruvate from the first stage (glycolysis) and sends acetyl CoA to the third stage (the Citric Acid Cycle).
Pyruvate decarboxylation is carried out by multiple copies of three different enzymes that are collectively called the pyruvate dehydrogenase complex (PDC). The first enzyme is called pyruvate dehydrogenase (E1), which uses thiamin to decarboxylate pyruvate into an acetyl group. The second enzyme is dihydrolipoyl transacetylase (E2), which attaches the acetyl group to co-enzyme A. This produces acetyl CoA. The third enzyme, dihydrolipoyl dehydrogenase (E3), uses both riboflavin (as part of FAD) and niacin (as part of NAD+) to separate acetyl CoA from the second enzyme.
Each of the three enzymes uses a permanently attached molecule called a prosthetic group to perform its task. E1 uses TDP, which contains thiamin. E2 uses a non-vitamin called lipoamide. E3 uses FAD, which contains riboflavin. Lipoamide isn't considered a vitamin because we can produce plenty of it ourselves. NAD+ is also involved, but it's not a prosthetic group like the others. It doesn't stay attached to an enzyme, but picks up its extra electrons and then takes them to the electron transport chain where they can be used to produce ATP. FAD and NAD+ both use their ring structures to transfer electrons, but you can think of FAD as the dock worker that loads up NAD+ with electrons, and NAD+ as the truck driver that transports them elsewhere.
While lipoamide is permanently attached to its enzyme like TDP and FAD, it's more mobile because it swings around like an arm. Its free end can transport molecules from one active site to another. Let's look at the entire process in more detail.
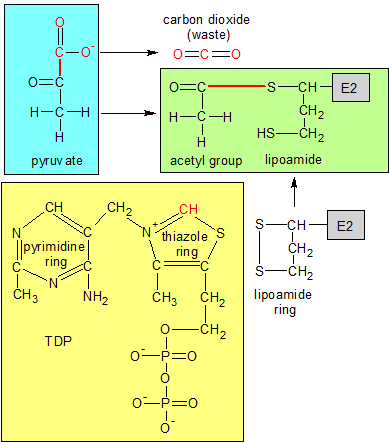
TDP (yellow) is embedded in E1 (not shown), with the red carbon atom sticking out. TDP's red carbon breaks the red bond in pyruvate, discarding CO2 and forming a temporary C-C bond (not shown) with the acetyl group. The new C-C bond reacts with lipoamide's ring on E2, transferring the acetyl group from TDP to lipoamide. This produces the green molecule. These reactions take place during the first two steps of the pyruvate decarb-oxylation process. |
The process begins when pyruvate (which is produced by glycolysis) arrives at the PDC and attaches itself to an active site on E1. TDP, which is also attached to E1, uses its thiamin ring to decarboxylate pyruvate. A section of pyruvate containing two oxygens and one carbon is severed from pyruvate, creating carbon dioxide and an acetyl group.
Step two takes place when the free end of the lipoamide prosthetic group on E2 swings over to E1 and reacts with E1's newly acquired acetyl group. The SS bond in lipoamide's ring is broken apart by the transfer of electrons from the CC bond that connects the acetyl group to TDP on E1. The acetyl group's carbon atom forms a covalent bond with one of the two sulphur (S) atoms, and is now bound to E2. TDP goes back to its original state.
In step three, E2 combines a nearby molecule of coenzyme A with the acetyl group to create a molecule of acetyl CoA. Acetyl CoA is the molecule that's used by the citric acid cycle to produce more ATP, but it's still attached to the sulphur atom on E2's lipoamide group. It must be detached before it can enter the citric acid cycle.
In step four the lipoamide group swings its attached acetyl CoA molecule over to E3, where FAD is waiting to reverse the reaction that took place in step two. Back in step two, lipoamide's ring was broken apart at the SS bond by adding electrons (reducing it). This was done in order to attach the acetyl group to one of the sulphur atoms. Now that we want to free acetyl CoA, we'll have to reverse that process by closing the lipoamide ring back up. FAD becomes FADH2 when it reacts with the CS bond that binds acetyl CoA to one of the sulphur atoms, and removes the hydrogen atom from the other sulphur atom. The two sulphur atoms then form an SS bond, which closes the lipoamide ring.
Acetyl CoA is now free to move about and enter the citric acid cycle, but we aren't finished yet. In the fifth and final step of pyruvate decarboxylation we need to remove the extra electrons from FADH2 before more acetyl CoA can be produced. FADH2 is a prosthetic group, so it can't leave E3 and dump its electrons off at the electron transport chain. That's where NAD+ comes in. With E3's help it uses its niacin ring structure to oxidize (remove the electrons from) FADH2, and then deposits them in the electron transport chain where they will be used to produce more ATP.
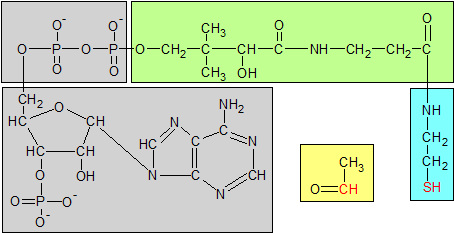
Coenzyme A has three parts. Phosphoadenosine diphosphate is gray, pantothenic acid is green and b-mercapto-ethylamine is blue. B-mercapto-ethylamine is the active part of coenzyme A. Its red sulphur atom binds to the acetyl group's red carbon atom to form acetyl CoA. The acetyl group is yellow. Pantothenic acid is a structural component of coenzyme A, not an active one. |
In the last section we saw how three of the B vitamins (riboflavin, thiamin and niacin) worked together to breakdown pyruvate into an acetyl group which then binds to coenzyme A to form a molecule of acetyl CoA. Pantothenic acid also participates in this process as a static componenent of coenzyme A. Because coenzyme A is involved in many biological processes, pantothenic acid is an essential nutrient.
Pantothenic acid is also used as part of a prosthetic group in a number of acyl carrier proteins (ACP), which synthesize fatty acids. Acetyl CoA and ACPs are used in almost all forms of life, so pantothenic acid is found in virtually all foods in both of these molecular forms. They are found in the highest concentrations in meat, avocado and the whole grains. Both of these molecules must be broken down in the intestine before the separated pantothenic acid can be absorbed.
Pantothenic acid works differently than some of the other B vitamins because it doesn't change during biological processes, but maintains a static substructure as part of the CoA and ACP molecules. Whether it's helping coenzyme A transport an acetyl group to the citric acid cycle during respiration or helping an acetyl carrier protein use an acetyl group to synthesize a fatty acid molecule, it performs the same function. It attaches one end of itself to a phosphate group on coenzyme A or ACP, and the other end of itself to the active b-mercapto-ethylamine molecule, which in turn binds to an acetyl group. We've already seen the process of how CoA combines with an acetyl group in the previous section, so this time I'll just show an image of how pantothenic acid fits into the overall structure of coenzyme A in the diagram above.
|
|
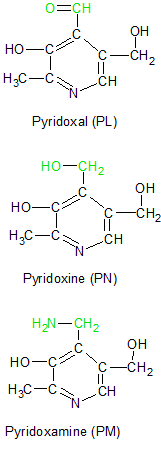
Vitamin B6 refers to a family of molecules that share a common ring structure. The three molecules vary only in their attached functional groups. Pyridoxine (PN), Pyridoxal (PL) and Pyridoxamine (PM) are the three unphosphorylated molecules, but each has a phosphorylated version abbreviated as PNP, PLP and PMP respectively. All six of these molecules can be converted into each other as needed, but the ring structure itself can't be assembled in people.
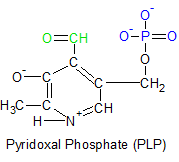
The three unphosphorylated forms of vitamin B6 are illustrated in the diagram on the left. Each of their functional groups are highlighted in green. Pyridoxine is generally considered the original form of vitamin B6, with the other two considered to be its vitamers. But it's the phosphorylated form of pyridoxal called pyridoxal phosphate (PLP) that's the most important member of the vitamin B6 family. PLP is shown on the upper right with its aldehyde group in green and its phosphate group in blue.

Potatoes contain lots of pyridoxine. |
Plants make pyridoxine, while animal-based foods mostly contain vitamin B6 in its pyridoxal and pyridoxamine forms. Pyridoxine is more stable than the other two, so less of it is lost during food processing. Its stability makes it the preferred form of vitamin B6 in vitamin pills. Many foods contain some vitamin B6, but meat, bananas and potatoes are especially high. Vitamin B6 is the most potentially toxic of the water soluble vitamins, but it's difficult to consume too much of it unless you are taking too many vitamin B6 supplements. Toxic levels can lead to conditions like nerve damage and skin lesions.
All three forms of vitamin B6 are absorbed from the intestine through passive diffusion, but the phosphorylated versions can't be absorbed until they are dephosphorylated by an enzyme called alkaline phosphatase. Once inside the cell, each molecule can be converted into the other two versions or phosphorylated (by pyridoxal kinase) as needed. The most biologically active form of vitamin B6 in humans is pyridoxal phosphate (PLP) because it has both an aldehyde (H-C=O) and a phosphate (PO4-2) group.
PLP functions mostly in the liver as a coenzyme in a wide variety of metabolic reactions. It's used by enzymes in the synthesis of neurotransmitters like serotonin and epinephrine, and in the synthesis of some lipids. It's also used in the creation of histamine and is central to the transfer of nitrogen from amino acids to other molecules in a process called transamination. The removal of an amino acid's alpha nitrogen group is a necessary first step before the nitrogen can be used elsewhere or eliminated from the body. It's also required before the rest of the amino acid can be broken apart for energy. Let's look at the overall process of transamination and then examine how PLP participates in that process.
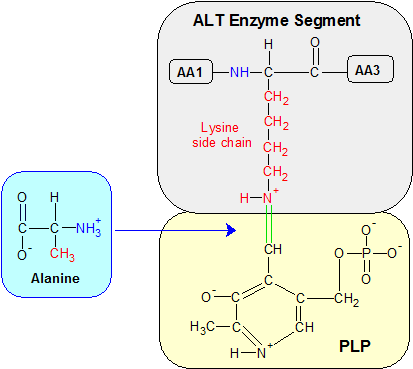
Step 1: Transamination begins when the green bond between PLP's carbon atom and lysine's epsilon amine group is severed in a hydrolysis reaction that restores PLP's aldehyde (H-C=O) group (not shown). Alanine binds to PLP's aldehyde group in a condensation reaction, forming the ketimine molecule shown below. The alpha amine groups are blue and the amino acid side chains are red. AA1 and AA3 are amino acids adjacent to lysine in the ALT enzyme. 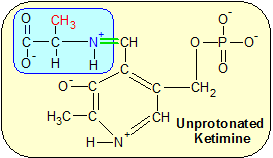
|
Transamination is a process that transfers an amine group (NH3) from an amino acid to an alpha-keto acid molecule. This can be useful in a variety of situations. If fats and carbohydrates are in short supply the carbon skeleton of the deaminated amino acid can be used as an alternative source of energy. The alpha-keto acid can then remove the remaining amine group from the body. If nitrogen is needed elsewhere the alpha-keto acid can donate its newly acquired amino group to other biological processes instead.
Transamination is used on all but two of the twenty kinds of amino acids that are used to build protein (lysine and threonine are deaminated into free ammonia instead). There are several different alpha-keto acids that are able to accept an amine group, depending on the amino acid involved. Each type of alpha-keto acid can accept an amine group from multiple amino acids, but no alpha-keto acid can work with all of them. This is because the enzymes that are used in transamination (collectively called transaminases) differ depending on the amino acid involved. To make sense of all of this, let me give you two specific examples of how all of this works.
Example 1: The aspartate transaminase (AST) enzyme transfers an amine group from the aspartate amino acid to a molecule of alpha-ketoglutarate. This produces oxaloacetate and glutamate. Now that aspartate has had its amine group removed, the resulting oxaloacetate molecule can be processed into glucose to supply energy. On the other side of the reaction, alpha-ketoglutarate has acquired the amine group and is now glutamate, a different kind of amino acid. Glutamate can keep its amine group and function as an amino acid, it can donate the amine group to other molecules as needed, or it can eliminate the amine group from the body entirely.
Example two: The alanine transaminase (ALT) enzyme transfers an amine group from the alanine amino acid to a molecule of alpha-ketoglutarate. This produces a glutamate amino acid from alpha-ketoglutarate (just like in the previous example), but the alanine amino acid becomes a molecule of pyruvate. Pyruvate can also be used for energy production as we saw earlier on this page when we looked at the pyruvate dehydrogenase complex.
One of the advantages of moving amine groups from other amino acids to glutamate is glutamate's ability to dispose of excess nitrogen. If aspartate and alanine's carbon skeletons (oxaloacetate and pyruvate respectively) are to be used for energy production, what do you do with the leftover nitrogen in the amine groups? Transferring it to alpha-ketoglutarate to produce glutamate solves this problem. An enzyme called glutamate dehydrogenase can remove (deaminate) the amine group from glutamate, converting it back into alpha-ketoglutarate. The unattached amine group (also called ammonia or NH3) then travels to the liver where it's eliminated from the body as urea.
Now that we've seen the value of transamination, let's see how vitamin B6 participates in this process. Transamination looks complicated because it involves specialized transaminase enzymes working with a variety of amino and alpha-keto acids. Underneath all of that complexity the specific reactions that involve vitamin B6 work the same way with all of these different molecules. Let's look at how PLP and transamination work in a general way and then examine a specific instance of the entire transamination process by using the molecules listed in example two in a step-by-step analysis with diagrams.
Step 2: The removal of a proton from the green hydrogen in the top image initiates a chain reaction that flips multiple bonds to their opposite states in a process called protonation. This moves the green double bond to the other side of the nitrogen atom. |
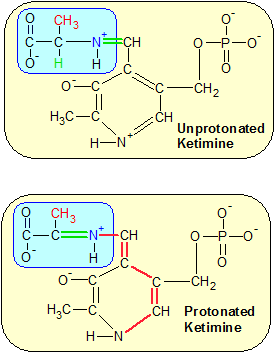
Step 3: Hydrolysis severs the green C=N+ bond again, but this time the double bond is on the other side of the nitrogen atom. Alanine is now a keto acid because the amine group was replaced with a keto group (the green double-bonded oxygen). PLP gained the amine group and is now Pyridoxamine phosphate (PMP). |
Pyridoxal phosphate (PLP) is the active form of vitamin B6 in all transaminase reactions due to the combination of its phosphate (PO4-2) and aldehyde (O=C-H) groups. The phosphate group keeps PLP positioned correctly in the enzyme's active site by forming hydrogen bonds with molecules in the enzyme, while PLP's aldehyde group is used to transfer an amine group from an amino acid to a keto acid (or back the other way, since it's a reversible reaction).
PLP's aldehyde group transfers amine groups to and from other molecules by using the same two reactions over and over again. A condensation reaction binds PLP's aldehyde group to another molecule's amine group by removing the oxygen atom from the aldehyde group and two nitrogen atoms from the amine group, which produces a molecule of water. This exposes two unpaired electrons on both the aldehyde's carbon atom and the amine's nitrogen atom, so they bind to each other. This produces a C=N+ double bond. A hydrolysis reaction severs the bond by reversing the process. One oxygen and two hydrogen atoms are added back in, which severs the C=N+ bond and restores the separate aldehyde and amine groups.
These condensation and hydrolysis reactions happen all the time to the C=N+ bond because the positive charge on the nitrogen atom makes the bond unstable around the highly polar water molecules that surround it. So how does forming and breaking the same bond over and over accomplish anything? That's where PLP and the transaminase enzyme come in. When the C=N+ bond is formed, there's another carbon atom on the other side of the nitrogen atom. Nitrogen can only form three bonds, so the other bond is always a single bond. It looks like C=N+-C. When the bond happens between PLP and an amino acid in the active site of a transaminase enzyme a reaction takes place called protonization. Here's how it works.
Step 4: An alpha-ketoglutarate keto acid's keto oxygen atom combines with PMP's amine group to form a new C=N+ double bond. A new protonated ketimine molecule is formed. |
The amino acid has a hydrogen atom attached to its center carbon atom. This hydrogen atom is positioned near a negatively charged area on the transaminase enzyme, which steals hydrogen's positively charged proton. Hydrogen is just a proton and an electron, so the remaining electron is now homeless. It travels from the amino (or keto) acid down to the positively charged nitrogen atom in the bottom of PLP's ring structure, flipping single bonds over to double bonds and vice versa along the way. This reverses the location of the double bond to the other side of the nitrogen atom in our C=N+-C bond. Under these new circumstances, when a hydrolysis reaction severs the C=N+ double bond, the nitrogen atom stays with PLP instead of the amino acid. Later, when PLP's stolen amine group forms a bond with a keto acid's oxygen atom the process reverses itself and the amine group is transferred again. That's how PLP makes transamination possible. Let's look at a specific step-by-step example with some helpful diagrams. We'll use the molecules listed earlier in Example Two.
Step One: We start the transamination process with PLP already bound to the epsilon amine group of a lysine amino acid in ALT's active site, as shown in the Step One diagram. The connection is a C=N+ double bond which is unstable due to the positive charge on the nitrogen atom. A water molecule severs the C=N+ bond in a hydrolysis reaction, restoring an oxygen atom to PLP's aldehyde group. The alpha amine group (NH2) on a passing alanine amino acid reacts with PLP's aldehyde group in a condensation reaction. Lysine remains part of the ALT enzyme while alanine and PLP form a new molecule called a ketimine.
Step two: This step begins with a restructuring of the ketamine molecule by a catalytic base in the enzyme's active site. The catalytic base is a molecule with a negative charge that steals the proton from the hydrogen atom that's attached to the central carbon atom on the alanine amino acid (now part of the ketimine molecule). It's the green H in the upper diagram of Step Two. The hydrogen atom's electron is left behind, and travels all the way down the ketimine molecule's structure to the positively charged nitrogen atom on the far side of ketimine's ring structure. This redistributuion of charge (called protonation) flips many of the single bonds into double bonds and vice versa. The significant change in this process occurs when the C=N+ double bond that connects alanine's amine group to PLP becomes a single bond, and the C-N+ single bond on the alanine side of the nitrogen atom flips to a double bond. This sets the stage for step three.
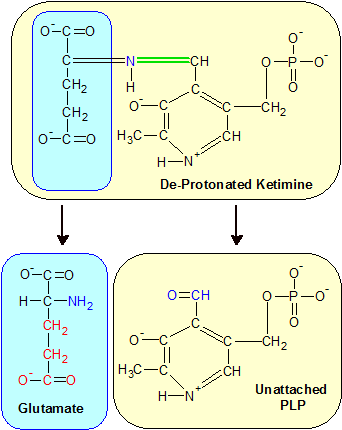
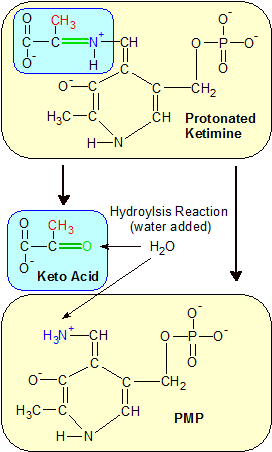
Step 5: The ketimine molecule deprotonates, moving the green double bond back to the PLP side of the molecule. Hydrolysis separates the two molecules again, but this time the amine group stays with alpha-ketoglutarate, producing an amino acid called glutamate. PMP reverts to PLP, which is shown here with its aldehyde group (blue) unattached to any amine group. |
Step three: This step begins with another hydrolysis reaction, but this time on the opposite side of the nitrogen atom. The ketimine molecule separates into two molecules as before, but this time PLP keeps the amine group instead of alanine. Alanine's exposed central carbon atom combines with an oxygen atom from a water molecule as part of the hydrolysis reaction to make up for its loss of the C=N+ double bond. The addition of the oxygen atom turns it into a keto acid, since a keto group is just another name for a double-bonded oxygen. PLP gains the nitrogen atom, which becomes an amine group as it collects two additional hydrogen atoms from the hydrolysis reaction. This transforms PLP into the amine form of vitamin B6 called pyridoxamine phosphate (PMP).
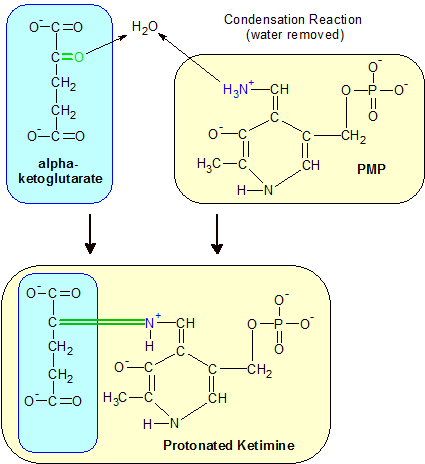
Step four: We focus our attention on PMP, which now holds the stolen amine group. At this point the process goes into reverse, but with a different alpha-keto acid. In this example we'll use alpha-ketoglutarate, which happens to be in the right place at the right time, but any keto acid will work. Alpha-ketoglutarate's keto group (a double-bonded oxygen) reacts with PMP's amine group in a condensation reaction. In this reaction the keto oxygen atom forms a water molecule with two of the hydrogen atoms attached to PMP's amide group, and the nitrogen atom in the amide group uses the two single bonds from its lost hydrogen atoms to form a double bond with alpha-ketoglutarate's exposed carbon atom. This is the same type of reaction we had in the second half of step one, and we now have a new ketimine molecule. Since we're using a different alpha-keto acid this time, we have a slightly different ketimine molecule.
Step five: In the fifth and final step, the new ketimine molecule deprotonates, reversing the earlier protonation of the ketimine molecule back in step two. This reverses the location of the C=N+ double bond back to the PMP side of the nitrogen atom where hydrolysis can once again separate the two molecules. PMP reverts to PLP with the loss of its nitrogen atom and alpha-ketoglutarate uses the nitrogen atom to create an amine group. This converts it into a glutamate amino acid. With its aldehyde group restored and no alpha keto groups nearby to react with, PLP re-establishes its bond with the lysine residue on the ALT enzyme and the process is complete.
Summary: When you combine the aldehyde group on PLP with the amine group on an amino acid you get a nitrogen atom attached to a carbon atom on either side. Nitrogen atoms form up to three stable bonds, so one side has a double bond and the other side has a single bond. The ring structure on PLP makes protonation possible, which shifts the double bond to the other side of the nitrogen atom. Since hydrolysis reactions break C=N+ bonds and condensation reactions create C=N+ bonds you can use these reactions in combination with protonation to move amine groups from one molecule to another. In our example we started with an alanine amino acid and ended up with a glutamate amino acid. That's just one of many biologically useful outcomes of transamination, and it's the ring structure of PLP that makes this process possible.
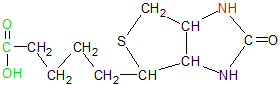
Biotin uses its carboxyl-tipped (green) tail to bind to a lysine residue on an enzyme and its red nitrogen to transfer carboxyl groups to other molecules. |
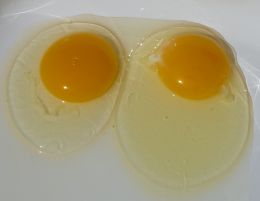
Egg yolks are a great source of biotin, but only if you cook the egg whites. Raw egg whites contain a protein called avidin that will bind to biotin, making it nutritionally unavailable. Cooking the egg white denatures the avidin. |
Bacteria in our large intestine produces biotin, but not enough to meet our needs. We get most of our biotin from the foods we eat. Our dietary biotin comes from a wide variety of both plant and animal based foods, but originates for the most part in plants, where biotin's unique double-ring structure is created. Plants (and bacteria) produce biotin by combining an alanine amino acid with a molecule of pimeloyl in a process that involves three different enzymes. The synthesis of these two molecules creates a vitamin with a double-ring structure and a long tail with a hydroxyl tip.
Biotin is found in a wide variety of foods, but in relatively low concentrations compared to the other B vitamins. Like all water soluble vitamins, biotin is absorbed from the intestine into the portal vein where it's transported directly to the liver. Most dietary biotin is bound to protein and must be separated from it in a process called proteolysis before the biotin can be absorbed into the body. The ratio of free to bound biotin is different depending on the food. Corn has more free biotin than any other grain. In some foods biotin is chemically bound in ways that our bodies can't separate. One interesting example is the egg. Eggs are especially high in biotin, but we can't use any of it if we consume the egg raw. This is because the biotin is bound to an avidin protein with an unbreakable bond. If we cook the egg, the avidin is denatured and the biotin doesn't bind to it.
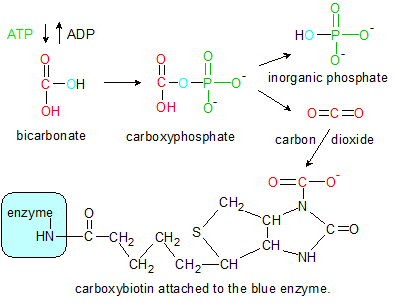
Step 1: Pyruvate carboxylase (blue) uses a phosphate group (green) from ATP to remove oxygen (blue) from bicarbonate. The hydroxyl group (red) becomes carbon dioxide before converting back into a hydroxyl group as it binds to a nitrogen on biotin's ring structure, forming carboxybiotin (black). |
Biotin acts as a prosthetic group for the enzymes, meaning it stays bound to an enzyme unless removed in a separate reaction. That's why most biotin in our food is already bound to a protein (enzyme) before we digest it. The bonds between biotin and most proteins can be severed by a protease enzyme during digestion. The biotin-aviden bond mentioned earlier is a rare exception.
Biotin participates in a large number of reactions. These include processes like gluco-neogenesis and the breakdown and synthesis of fatty acids. Biotin is able to transfer carbon atoms from one molecule to another. It does this when the carbon atom is in its most oxidized form. This means it's bound to two oxygen atoms. When the oxidized carbon atom is bound to a molecule (usually to another carbon), the carbon atom is part of a single-carbon group called a carboxyl (HO-C=O). When disconnected, the single-carbon group becomes a molecule of carbon dioxide (O=C=O), which converts back into a carboxyl group when it arrives at the destination molecule.
In the following example we'll see how biotin transfers a carboxyl group from a bicarbonate molecule to pyruvate in order to form oxaloacetate. Pyruvate carboxylase is the enzyme that oversees this transfer, but biotin must be bound to a lysine residue in the enzyme's active site in order for the reaction to take place. The carboxyl group on the end of biotin's tail binds to lysine's amine group in a condensation reaction that results in a C-N bond between biotin and the enzyme. This creates a long chain of atoms that connect the ring structure on biotin to the enzyme, where it remains during the entire process. The long chain acts like a tether, giving biotin's ring structure the ability to swing between multiple active sites on the enzyme. Let's see what happens when all of these molecules are in the right place at the right time.
Step 1: The transfer of a carboxyl group to pyruvate begins at the first of pyruvate carboxylase's two active sites. The enzyme uses energy and a phosphate group from a molecule of ATP to transfer a carboxyl group from a molecule of bicarbonate to biotin. This is a multi-part process that begins with the hydroxyl group from bicarbonate combining with one of ATP's phosphate groups to form a molecule of carboxyphosphate. Carboxyphosphate then breaks down into inorganic phosphate and carbon dioxide (CO2). The energy used from ATP to create carboxyphosphate is released during carboxyphosphate's breakup, and used to bind the carbon dioxide to biotin's nitrogen atom. The enzyme's role is to position all of the molecules so that the energy transfer can take place.
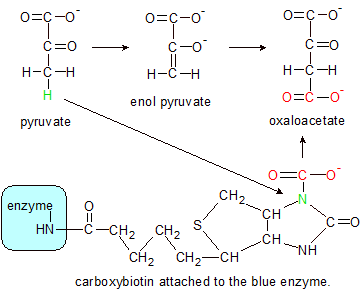
Step 2: Pyruvate's green hydrogen atom binds to carboxybiotin's green nitrogen, which severs the C-N bond, converting carboxybiotin back into biotin. Pyruvate takes on an enol form until it combines with the severed red hydroxyl group, which creates a molecule of oxaloacetate. |
Note the blue oxygen atom in the Step One diagram. ATP adds a phosphate group with three oxygens to bicarbonate, but removes the four oxygen (inorganic) version of phosphate from caroxyphosphate. This removes the extra oxygen from bicarbonate, converting it to carbon dioxide. The carbon dioxide then binds to biotin's nitrogen atom as a carboxyl group. The end result is a molecule called carboxybiotin.
Step 2: This step begins when the unattached end of carboxybiotin (containing the carboxylated biotin ring structure) swings over to the enzyme's second active site where it reacts with pyruvate in another multi-part process. Pyruvate donates a hydrogen atom (green in the step 2 diagram) to biotin's green carboxylated nitrogen atom. This severs the C-N bond, restoring biotin to its original uncarboxylated state and converts pyruvate into an enolate form. The severed carboxyl group (red) converts to a molecule of carbon dioxide (not shown), which then converts back into a hydroxyl group as it binds to the end of the enolate form of pyruvate where the hydrogen atom used to be. This converts pyruvate into a molecule of oxaloacetate. Its job complete, biotin then swings back to the first active site where it starts the process all over again.
Why is the conversion of pyruvate into oxaloacetate important? Oxaloacetate is an intermediate molecule in many important biological reactions, including the citric acid cycle and fatty acid synthesis. The energy taken from ATP in step one is transferred into oxaloacetate's additional C-C bond (relative to pyruvate) where it can be re-directed into other processes or stored for future use. If the energy isn't needed right away it can be used for fatty-acid synthesis, a process that transfers stored energy from carbohydrates into the much more efficient (and longer-term) energy storage molecule called fat. This is just one of the ways in which biotin participates in the processes that keep us healthy.
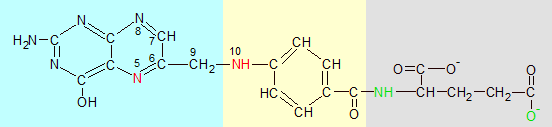
Folic acid (above) is made up of pterin (blue), p-aminobenzoate (yellow) and glutamate (grey) sections. Tetrahydrofolate (THF) (below) is folic acid with four extra hydrogens in the pterin ring and additional glutamate groups added to its gray end. The red nitrogen atoms grab single-carbon groups when they are released from amino acids by enzymes. 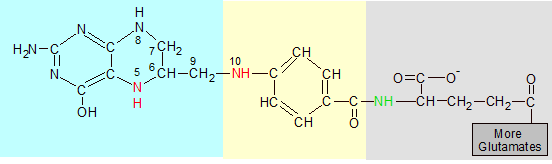
|
Folic acid is a combination of three structures. Pterin and p-aminobenzoic are combined to form a pterin-p-aminobenzoic acid intermediate (PABA). Glutamate (an amino acid) is then added to the molecule. Folic acid is the molecule that's called vitamin B9, but it's not biologically active until it becomes a folate.
Folates are a family of molecules that are derived from folic acid, but may exist in any number of altered states brought about by the partial or complete reduction of folic acid (by adding hydrogen atoms), or the binding of additional glutamates. Animals (including humans) can convert folates from one state into another, but we can't synthesize the pterin ring structure from scratch. Plants are able to do this, and provide pre-assembled folates in our diet. The most biologically significant folate is called tetrahydrofolate (THF), so I'll use it to describe the two types of changes that occur during the transformation of folic acid into a folate.
The first change is the bonding of four hydrogen atoms to the two nitrogen and two carbon atoms (labeled 5-8 in the diagram) on the right side of the pterin double ring. This reaction is catalyzed by the dihydrofolate reductase (DHFR) enzyme. The hydrogen atoms are donated by two NADPH and two protons (H+). This reaction sets up the nitrogen at position 5 to bind to a methyl (CH3) group.
The second change is the binding of more glutamates onto the end of folic acid's pre-existing glutamate. There are several variations of THF that differ only in the number of attached glutamates, but the biologically active versions usually contain five or six glutamates. These are used to attach THF to an enzyme and position it where it can be useful. You can see before and after images of folic acid and THF in the image above.

|
Folic acid isn't biologically active until it's converted into one of its folate forms, so plants usually convert it into a folate for their own use before we consume it. This may sound like a good thing, but we can't absorb folates into our bodies due to their long glutamate tails. Enzymes in our small intestine have to remove all but one of the glutamates and attach a methyl (CH3) group to the end of the remaining glutamate before it can be absorbed. Folic acid and the single-glutamate folates are the form in which vitamin B9 is absorbed and transported. The multi-glutamate folates are the form in which vitamin B9 is stored and biologically active.
Folates are produced in bacteria and plants, with especially high concentrations in avocado, liver and the leafy greens. Folic acid is added to some grain products and many vitamin supplements (instead of the folate forms) because its single glutamate structure sidesteps the absorption problem. Most folic acid is converted into the desired folate (usually THF) in the liver. Excess folate is also stored there, which is why liver is such a good source of dietary folate. Some excess folate is also combined with bile and excreted into the small intestine, where it's reabsorbed back into the body for reuse. In this way vitamin B9 is recycled as needed.
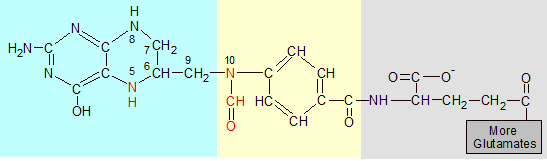
N10-formyl-THF is THF with the red formyl group (O=CH) attached to N10. To get 5,10-methyl-THF (not shown) the oxygen atom is removed and both N5 and N10 bind to the methyl group's (CH2) carbon, forming an additional ring. |
What makes folate so important? It's involved in the transfer of single carbons from one molecule to another as a methyl (CH3), formate HCOO-) or formaldehyde (OCH2) group. These transfers are a necessary part of many processes, including DNA synthesis. If new DNA can't be produced, cells postpone division. The mature cells grow larger than normal because they have trouble dividing. If they do divide, they are usually damaged. This is especially evident with red blood cells because they have a short life-span and must reproduce more often than other types of cells. When bone marrow can't produce enough red blood cells due to a folate shortage it releases extra-large damaged red blood cells into the bloodstream. This produces an illness called pernicious anemia. Let's see how THF participates in the synthesis of DNA.
THF removes the red formyl group (OCH) from the serine amino acid, leaving glycine (in black) behind. |
DNA consists of a long string of nucleotides. THF is involved in the synthesis of these nucleotides through the transfer of single-carbon groups from some amino acids (usually serine or glycine), to a variety of nucleotide precursors. THF can work with multiple enzymes and substrates, but in this example we'll use serine hydroxy-methltransferase (SHMT) for our enzyme, and a serine amino acid as the source of our single-carbon group.
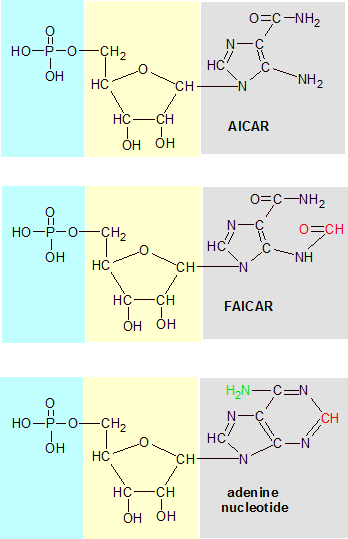
N10-formyl-THF (not shown) transfers a formyl group (O=CH) to AICAR, transforming it into FAICAR. The oxygen is discarded later, but the carbon remains as a permanent addition. The first two images are intermediate molecules with the purine double-ring structure under construction. The third image is an adenine nucleotide. Its nitrogenous base (gray) is a completed purine structure with an extra nitrogen atom (green) added to make it adenine. The phosphate (green) and deoxyribose (yellow) areas are the same in all three images. |
SHMT begins the process when it uses another B vitamin (B6 in the form of PLP) to bind to the serine amino acid. This process is similar to the one used on alanine in the earlier B6 article. PLP binds to serine's nitrogen atom, forming a C=N double bond. PLP then pulls an electron from serine down into PLP's ring structure, which reverses the single and double bonds along the way, just as with alanine. But here's where the story changes. Instead of the C=N double bond being severed as it was during transamination in the alanine-B6 example, SHMT severs a C-C bond on serine instead. This splits serine into a glycine amino acid and an OCH2 formaldehyde molecule.
THF is pre-positioned in SHMT's active area and scoops up the single-carbon formaldehyde molecule, binding with it in a condensation reaction that releases the oxygen atom as water, converting the formaldehyde into a methyl group. THF's two active nitrogen atoms (N5 and N10 in the diagram) bind to either side of the carbon atom, forming a molecule of 5,10-methylene-THF. From this point the single-carbon group can separate from nitrogen 5 and oxidize into 10-formyl-THF or separate from nitrogen 10 to become 5-methyl-THF, depending on the process involved. In our example we'll stick with 10-formyl-THF, because that's the form that participates in DNA synthesis.
Vitamin B9 is critical to DNA synthesis because N10-formyl-THF molecules donate their formyl groups during two different steps in the creation of a purine double-ring structure. This structure is a necessary precursor for the synthesis of adenine and guanine (two of DNA's four nucleotides). DNA synthesis has many steps, most of which have nothing to do with vitamin B9. To see every step of the process that uses vitamin B9 (as N10-formyl-THF), search for information on "purine nucleotide biosynthesis". Since this is an article about vitamin B9 rather than DNA synthesis, I'll give you an isolated example of the second of the two steps in DNA synthesis where a N10-formyl-THF molecule contributes a single-carbon group to the construction of a purine ring structure. But before that, here's a paragraph on the structure of DNA.
DNA is a string of four different nucleotides (adenine, guanine, thymine and cytosine) that are strung together in different patterns to store genetic information. All four nucleotides are made of three sections: A phosphate group, a deoxyribose sugar, and a nitrogenous base, which defines which kind of nucleotide it is. Two of the nucleotides (adenine and guanine) contain a double-ring nitrogenous base called a purine. It's this purine structure that can't be constructed without the help of N10-formyl-THF.
During the creation of the purine double-ring structure, an intermediate molecule called AICA ribonucleotide (AICAR) is produced. One of the carbons in its structure was donated by a N10-formyl-THF molecule earlier, but now it needs an additional carbon atom to move on to the next step in the process. An enzyme called AICAR transformylase transfers the formyl group from N10-formyl-THF to AICAR to create another intermediate molecule called FAICAR. N10-formyl-THF reverts to THF in the process. This is just one example of how vitamin B9 transfers a single-carbon group to another molecule.
Plants have no use for vitamin B12, so most of them (with a few minor exceptions) don't produce it. Animals need it, but they can't make it on their own. Bacteria are the only form of life that can synthesize vitamin B12 from scratch. Some of the bacteria in the human intestine are able to create B12, but we can't absorb any of it because it's produced in our large intestine, where it can't be absorbed. Vitamin B12 is absorbed only in the lower part of the small intestine called the ileum. Except for supplements and fortified foods like breakfast cereals, almost all of our dietary B12 comes from animal-based products like meat, fish, eggs and dairy. All of these sources ultimately originate with bacteria.

Animals add vitamin B12 to milk and eggs for their young, and humans add it to their breakfast cereals. All three are good sources of vitamin B12, but nothing beats a slice of liver. |
One group of animals (called ruminants) have complex digestive systems that absorb B12 from the bacteria in their own gut. This is where much of our vitamin B12 enters the food chain. Ruminants are animals like cows and sheep, which pass vitamin B12 on to us in the form of meat and dairy. Other animals may eat insects, which in turn eat bacteria or manure. Manure contains vitamin B12 because it was produced in an animals large intestine by bacteria. For example, chickens eat worms, and worms eat bacteria. Lots of animals eat insects, and lots of insects eat manure. Such are the humble origins of a healthy diet.
Once vitamin B12 is absorbed into our bodies it's used wherever it's needed. Mammals put it into milk and chickens put it in their egg yolks. All animals store significant amounts in their muscles, so meat is a good dietary source of B12. Most vitamin B12 is stored in the liver, so liver is the most highly concentrated source of B12.
Vitamin B12 is a collection of nearly identical molecules that all contain a common base called cobalamin. The members of this family (which we'll look at later) differ in the addition of a variety of active groups. Cobalamin is a large molecule that's usually bound to a protein in the foods we eat. This means it needs a lot of help getting into our bodies where it can do us some good. When cobalamin reaches our stomach we use hydrochloric acid and an enzyme called pepsin to separate cobalamin from the protein it's bound to. It then binds to a glycoprotein called intrinsic factor (IF) that's secreted by the stomach. Cobalamin is then absorbed by receptors in the lower small intestine that can't absorb it unless it's bound to intrinsic factor.
Once inside the body, vitamin B12 is the easiest of the water soluble vitamins to store and reuse. Most of its biological activity is in the liver, so that's where it's stored in the highest concentrations. There's also significant amounts in muscle tissue and lesser amounts in most other cells. It's also recycled over and over due to a process called enterohepatic circulation. In this process excess cobalamin is added to bile, which is deposited into the small intestine for elimination from the body. The cobalamin in the bile combines with intrinsic factor and is reabsorbed instead of eliminated. B12 Deficiencies are usually due to problems with absorption of cobalamin, not a lack of it in the diet. An exception to this would be people (like vegans) who avoid all animal-based foods, since vitamin B12 is found almost exclusively in animal-based products. A prolonged deficiency of vitamin B12 can result in a disease called pernicious anemia.
|
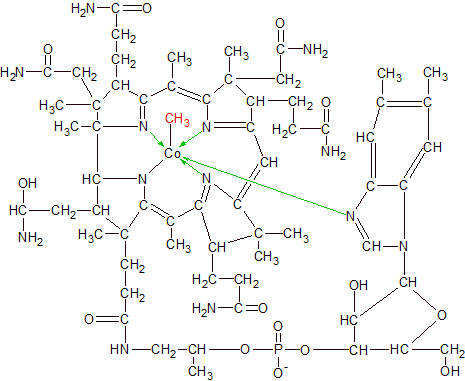
Cobalamin has the most complex structure of all the vitamins. It has a cobalt (Co) atom bound to the center of a multi-ring structure with many additional attached groups. The cobalt atom is bound to the structure by six covalent bonds, four of which are coordinate bonds. A covalent bond occurs when two atoms each share one unpaired electron in order to form an electron pair. A coordinate bond is a covalent bond where one atom provides both of the electrons for the pair. In cobalamin's case all four of its coordinate bonds are formed when four nitrogen atoms each donate an electron to the cobalt atom and then form a covalent bond with it.
Nitrogen normally contains five electrons and three empty spots in its outer shell, so it usually forms a maximum of three covalent bonds. By donating an electron to cobalt, nitrogen has four electrons and four empty spots, and can now form four covalent bonds. Cobalt starts with only two electrons in its large outer shell, so it can accept four donated electrons from other atoms and still have more than enough space left to form six covalent bonds. One of the two non-coordinate covalent bonds connects cobalt to a fifth nitrogen atom, while the sixth covalent bond connects it to its active group, which varies depending on which vitamer of cobalamin it happens to be.
Vitamin B12 and cobalamin don't refer to a single molecule, but to a family of structurally similar molecules that differ only in the group that's attached to cobalt's 6th covalent bond. Diagrams of cobalamin usually show an "R" where the active group is located to indicate there are multiple possibilities for the atoms located at that position. A cyano (CN) group makes it cyanocobalamin, a hydroxyl (OH) group makes it hydroxocobalamin, and a methyl (CH3) group makes it methylcobalamin. A much larger deoxyadenosyl active group makes it adenosylcobalamin. We'll focus on methylcobalamin in our example of how vitamin B12 works.
|
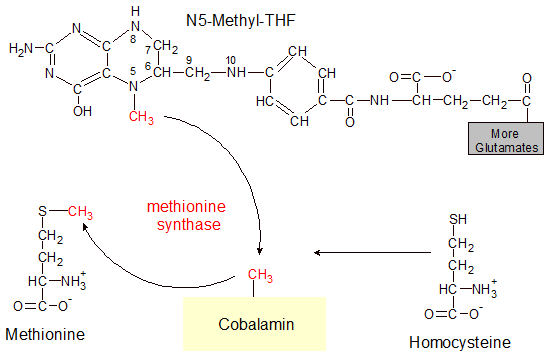
In our example of how cobalamin works, an enzyme called methionine synthase transfers a methyl (CH3) group from a molecule of N5-methyl-THF (one form of vitamin B9) to cobalamin, producing a molecule of methylcobalamin. This is an intermediate step in the transfer of a methyl group to homocysteine, which converts it into methionine. Methionine is one of the twenty amino acids that are used in protein. Methionine synthase can't transfer the methyl group directly from N5-methyl-THF to homocysteine, so cobalamin acts as an intermediate transfer point.
This may seem like a very small task for such a large molecule, but it's an important step in the creation of methionine. It's also the only way that vitamin B9 can be recycled once it gets into its 5-methyl-THF form. In the previous section on vitamin B9 we saw how it can take on multiple activated forms like N10-formyl-THF, 5,10-methyl-THF and N5-methyl-THF.
While the other two forms of vitamin B9 can reverse themselves back into inactivated THF or participate in reactions that don't need cobalamin, the N5-methyl-THF form of vitamin B9 is a non-reusable dead-end without any assistance from cobalamin. This is why cobalamin is needed in the diet to prevent pernicious anemia. While vitamin B9 prevents pernicious anemia by participating in DNA synthesis, it can't be used in this way until it can get rid of its N5 attached methyl group and reactivate as N10-formyl-THF. The transfer of the N5 methyl group not only activates cobalamin, but also re-activates vitamin B9 by freeing it to receive another methyl or formyl group from an amino acid.
|
Most plants and animals use an enzyme called L-gulonolactone oxidase to convert simple sugars into ascorbic acid. Ascorbic acid doesn't fit the definition of a vitamin for them since they can produce all they need on their own. Plants convert mannose and galactose into ascorbic acid, and most animals do the same thing with glucose. Human beings and some other creatures aren't so lucky. At some point in our evolution the gene that contains the instructions for creating L-gulonolactose oxidase mutated, and doesn't work anymore. We must now get ascorbic acid from our diet.

|
 |
Ascorbic acid is very similar to the fructose monosacharride in form, but not function. They are both made exclusively from carbon, hydrogen and oxygen atoms that are arranged into a five-sided ring structure with a few attached atoms. Fructose has a chemical composition of C6O6H12 while ascorbic acid has a composition of C6O6H8. Ascorbic acid has a slightly different structure and four less hydrogen atoms than fructose, and that's all it takes to change its functional role in the body. Unlike fructose, ascorbic acid is a powerful reducing agent. This means it can donate electrons to other molecules.
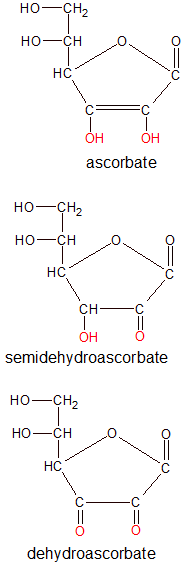
Vitamin C molecules fluctuate between three structural forms as they participate in biological processes where they donate one or two electrons. The forms are ascorbic acid (ascorbate), semidehydroascorbic acid (semidehydroascorbate) and de-hydroascorbic acid (dehydroascorbate). Ascorbic acid is the reduced form of vitamin C (has both of its electrons) and dehydroascorbic acid is the oxidized form (gave up both electrons). Semidehydroascorbic acid has donated only one of its two electrons.
Vitamin C can be found in a wide variety of plant-based foods, but some of the best sources include citrus, strawberries and brussels sprouts. Vitamin C is destroyed by too much heat and diffuses into surrounding water during preparation, so foods are highest in vitamin C content when they are raw and unprocessed. Deficiencies of vitamin C can result in a disease called scurvy. There are no serious side effects when taking large amounts of vitamin C, but the body can absorb only so much. Most of what is consumed when taking megadoses of vitamin C supplements is passed out of the body unused.
Vitamin C complements vitamin E as an antioxidant. Vitamin E is a fat soluble vitamin, so it neutralizes free radicals in fatty tissues where the water soluble vitamin C has trouble getting to. Vitamin C, on the other hand, can neutralize free radicals in watery environments like the bloodstream where vitamin E is at a disadvantage. In addition, vitamin C (as ascorbate) is one of the molecules that reduces vitamin E so that it can be reused as an antioxidant once again. It does this by transferring an electron to vitamin E at the lipid-water membrane interface where they can meet. Ascorbate oxidizes into semidehydroascorbate as a result. The enzyme semidehydroascorbate reductase then reduces semidehydroascorbate back into ascorbate. For more details on how antioxidants work, see the article on vitamin E .
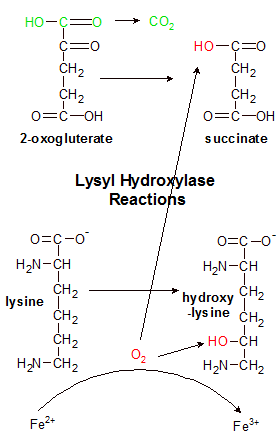
Lysyl hydroxylase uses iron (Fe2+) to split O2 into two red hydroxyl (OH) groups. It uses one group to hydrolyze lysine. It removes the green part of 2-oxogluterate and replaces it with the other OH group, creating succinate. The process can't hydrolyze another lysine amino acid until vitamin C reduces Fe3+ back to Fe2+. |
In addition to its role as an antioxidant, vitamin C participates in many other important biological functions. Ascorbate works with enzymes that use metal atoms like iron and copper by keeping those metals in a reduced state. This ability also helps with the absorption of iron into the body from the small intestine. Vitamin C helps to synthesize many different molecules by working with a wide variety of enzymes. One of these molecules is collagen.
Collagen is a compound that's made from the interweaving of multiple strands of protein. It forms a connective structure that holds the tissues of the body together. When vitamin C isn't available, collagen production is compromised. The existing collagen gradually disintegrates and isn't replaced. Wounds that require collagen in order to be repaired are no longer able to heal, and body tissues literally start to come apart. This condition is called scurvy. Vitamin C prevents scurvy by participating in the production of collagen, so examining how collagen is produced will be a good way to see how vitamin C works.
Collagen is produced by weaving together three proteins (called alpha chains) into a triple helix structure. There are many different kinds of alpha chains that combine to form a variety of collagen structures for use in different types of tissues in the body. The rigid structure of collagen holds the tissues of the body together.
Before the three individual alpha chains can be woven together and bound to each other by hydrogen bonds, proline and lysine amino acids in the alpha chains have to be hydrolyzed. Hydrolysis of these two amino acids enable the three strands to twist into a triple helix structure. Enzymes called prolyl-3-hydroxylase and prolyl-4-hydroxylase convert proline into two variations of hydroxyproline called 3-hydroxyproline and 4-hydroxyproline. Another enzyme (lysyl hydroxylase) converts lysine into hydroxylysine. Hydroxyproline's ring structure provides bulges in each strand that the other two strands can wrap themselves around. This increases hydrogen bond formation between the chains and enhances the triple-helix structure's stability. Hydroxylysine uses its newly aquired hydroxyl (OH) group to bind to glucose or galactose in a glycolysis reaction. The addition of either monosaccharide forces the strands to twist, which creates the triple helix structure.
Vitamin C participates at the end of the hydrolysis reaction. The enzymes can hydrolyze an amino acid once without the help of vitamin C, but can't hydrolyze another amino acid until vitamin C restores an iron atom to its original state. Let's see how vitamin C (ascorbate) is used to reduce iron after lysyl hydroxylase converts a lysine amino acid to hydroxylysine.
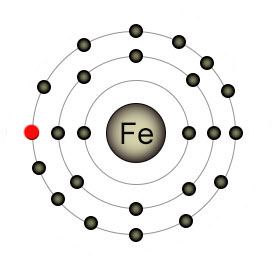
Iron in the form of (Fe2+) has a full outer shell. When lysyl hydroxylase removes the red electron it becomes Fe3+. It reacts with ascorbate to refill its outer shell and ascorbate becomes semihydroascorbate as a result. |
The active site in lysyl hydroxylase is a crowded place. It contains an iron atom (Fe2+), an alpha chain that contains lysine, and a few other molecules (ascorbate, O2, and 2-oxoglutarate). The goal here is to hydroxylate lysine by adding an OH group to it. The O2 molecule has two oxygen atoms, but only one is needed, so the O2 double bond has to be broken. Lysyl hydroxylase is a type of enzyme called a dioxygenase. This means that it finds a home for both oxygen atoms in the O2 molecule. Lysine only needs one, so the other will go to 2-oxoglutarate to form succinate.
Lysyl hydroxylase begins the process by decarboxylating 2-oxoglutarate, discarding the severed fragment as CO2. It then uses an iron (Fe2+) atom to break the O2 molecule apart. One oxygen atom is bound to the remains of 2-oxoglutarate, forming a molecule of succinate, which is also discarded. The other oxygen atom (as OH) binds to lysine in a hydroylsis reaction that produces hydroxylysine. The iron atom started the reaction as (Fe2+), but lost an electron in the process and is now (Fe3+). This is where ascorbate comes in.
The enzyme can't hydroxylate another lysine until the iron atom replaces its lost electron. Ascorbate contributes an electron to iron, reducing it to Fe2+. Ascorbate can donate one or both electrons, so it can participate in up to two reactions before it needs to be recharged (reduced). If it donates one electron it becomes semidehydroascorbate, which is reduced back to ascorbate by semidehydroascorbate reductase in a reaction that transfers an electron from a molecule of NADH. If both electrons are donated it becomes dehydroascorbate, which is reduced back to ascorbate by dehydroascorbate reductase in a reaction that transfers two electrons from a molecule of glutathione.
Many biological reactions involve electron transfers, and this is just one example of how vitamin C participates in some of them. Transferring electrons is all that vitamin C does, but it's enough to make it an important vitamin and an essential nutrient.