
Garden and Plate
The Molecular Biology of Nutrition










Four of the thirteen vitamins are fat soluble. They are non-polar or mostly non-polar molecules that have to combine with other molecules in order to move through watery environments like the bloodstream. This is because polar molecules (like water) attract each other, squeezing non-polar molecules out of the environment in the process. For this reason the fat-soluble vitamins tend to end up in fatty tissues like cell membranes or stored in fat cells until they are used. This makes them easier to store in the body than the water-soluble vitamins, but also easier to reach toxic levels with if consumed in large doses through supplements. I look at each of these vitamins in detail below.
|
Vitamin A performs a wide variety of functions in the human body. It works with hundreds of enzymes in the maintenance of clear vision, a strong immune system, and healthy skin. It also controls cell growth and differentiation by binding to DNA in a way that influences gene expression. Vitamin A takes the form of three different molecules that are collectively referred to as the retinoids. All three of these are ultimately derived from a few carotene molecules that are only produced in plants.
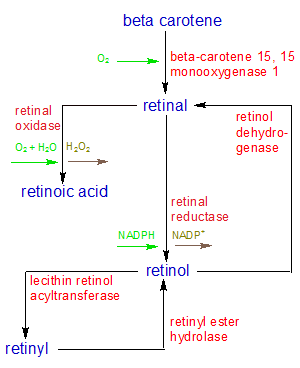
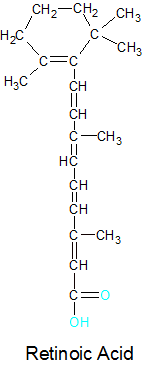
The three molecules that make up the vitamin A family are retinal, retinol, and retinoic acid. We consume beta carotene from plants and split it into retinal. The retinal is then used as it is for the maintenance of vision or converted into retinoic acid for other uses. If the retinal isn't needed right away, it's converted into retinol for storage. All three retinoids are unstable, so retinol must combine with one of several acidic molecules to form a stable molecule called retinyl. This is how vitamin A is stored in the body for future use. This reaction is reversable, so the retinol can be separated from the acid and used to make retinal or retinoic acid as needed. Retinol has no useful biological function other than as a storage and transport form of vitamin A.
We can't produce vitamin A from scratch, so we must get it in the food we eat. We consume most of it in two forms. We get carotenes (mostly beta carotene) from the plants we eat, and convert it into retinal. Since other animals do the same, we get retinoids from the meat we eat. Since the retinoids are stored as retinyl, most of the retinoids we consume from meat is consumed as retinyl. Over 90% of retinyl is stored in the liver, so eating liver is a good way to get your Vitamin A.
As you can see, vitamin A is more than a single molecule that performs a single function. It's a whole family of related molecules that do many things. The relationships between the molecules in the vitamin A family are shown in the flowchart on the left. I examine the molecules and the functions they perform in more detail below. Since all of the vitamin A molecules are derived from the carotenes, I'll start by looking at how and why the carotenes are created in plants. Then I'll show how they are converted into each of the retinoids in animals, and how they are stored as retinyl. I'll also describe what each of these three forms of vitamin A do in our bodies.
|
Carotene gets its name from the carrot, which derives its orange color from beta carotene molecules. There's also beta carotene in green leaves, where it assists with photosynthesis. The leaves are green instead of orange because chlorophyll is able to reflect different wavelengths of light. This is why most leaves look green. There's no chloro-phyll in the roots, so they're orange. 
Why do carrots place beta carotene in their roots if roots are in the dark and don't do photosynthesis? We did that. Carrot roots weren't always orange. They used to be purple. Hundreds of years ago farmers selected varieties that stored beta carotene in their roots because they liked the orange color. |
The carotenes are a group of molecules that are created by plants. They are useful because they contain beta-ionone rings that are able to selectively absorb specific wavelengths of light and change their molecular shape as a result. They are used by plants to add color to flowers and fruit, and to transfer the energy collected by chlorophyll during photosynthesis.
All carotenes are composed of nothing but carbon and hydrogen atoms, so they are hydrocarbons. They all have forty carbon atoms, but differ from each other in the number and/or location of their attached hydrogen atoms. Plants create them by combining eight isoprenes (C5H8) into a single molecule. None of the carotenes are fully saturated with hydrogen atoms, so double carbon (C=C) bonds exist in all of them, but at different locations. This gives them different characteristics. Some of them have one beta-ionone ring, while others have two. Because they contain only carbon and hydrogen atoms, they are non-polar molecules. This means they avoid water and are attracted to fat molecules, making them fat soluble.
The carotenes as a group can provide a wide variety of valuable biological functions, but only a few can be converted into Vitamin A. While alpha and gamma carotene can be converted into Vitamin A, most of what animals produce is derived from beta-carotene, so we'll focus on this particular molecule.
Animals can't create beta-carotene, but they can store it in body fat or the liver for future use. They aquire it when they eat plants. Beta carotene can be absorbed from the intestine whole, or split into two retinal molecules and then absorbed. The beta-carotene molecule is split into two twenty-carbon molecules of retinal in the liver or small intestine by the beta-carotene 15,15'-monooxygenase 1 (BCMO1) enzyme. This enzyme uses an O2 molecule to split the C=C double bond in the center of the beta-carotene molecule, attaching an oxygen atom to the newly exposed carbons at each end.
Both beta carotene and retinal can be stored in the body, but it's preferable to store your vitamin A as beta carotene. Beta carotene can be converted into retinal, but retinal can't be converted back into beta carotene. This can be a problem if you consume too much retinal, because retinal can be toxic in high concentrations. Retinal must be converted into retinol for storage, but this doesn't help because it is also toxic in large amounts. Beta carotene is never toxic, no matter how much you store. The worst that can happen is that it will turn your skin orange. That's why vitamin pills usually include part of their "vitamin A" supplement as beta carotene. The human body has no direct use for beta carotene. It's only useful once converted into retinal.
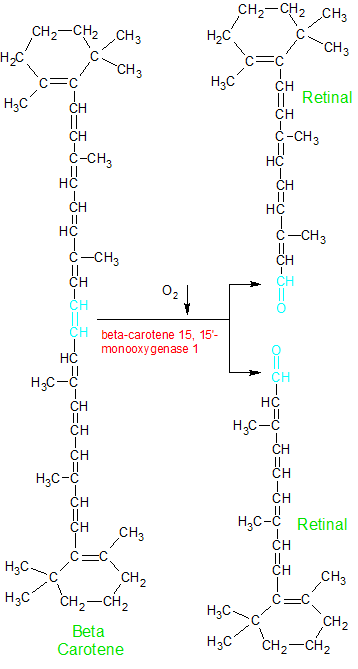
The enzyme beta-carotene 15,15-monooxygenase 1 uses an O2 molecule to split beta carotene into two molecules of retinal. Note retinal's aldehyde group (shown in blue). |
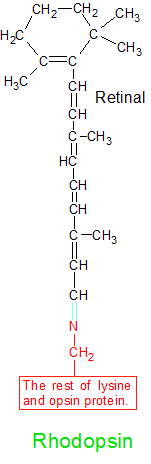
Retinal (aka retinaldehyde) can be converted into retinol or retinoic acid, but it's also very important in its current form. The aldehyde (CHO) group that's created on one end of retinal when beta carotene is split in half is able to combine with an opsin protein to create a molecule of rhodopsin.
An image further down the page shows part of the structure of the rhodopsin molecule. Retinal's aldehyde group combines with a lysine amino acid (the red portion) on the opsin protein in a condensation reaction that creates a rhodopsin molecule. Retinal's oxygen atom is discarded, and its terminal carbon atom forms a double bond with lysine's nitrogen atom.
Rhodopsin is important because of its ability to transform light into a signal that is sent to the brain. In other words, it gives us the ability to see. With one end of retinal covalently bound to the opsin protein, the other end (which is a light sensitive beta-ionone ring) is ready to react to light. When light is absorbed by the beta-ionone ring, retinal changes its shape. This causes the opsin protein to also change its shape, which in turn sends a signal to the brain. This is how we see, and why eating carrots (or any retinoid or beta carotene rich food) is good for the eyes. A severe lack of vitamin A in the diet causes blindness in many people every year.
Retinal can be converted into another retinoid called retinoic acid. An enzyme called retinal oxidase uses oxygen (O2) and water (H2O) molecules in a reaction that binds a second oxygen atom to retinal's terminal carbon atom. The product is retinoic acid and a discarded H2O2 molecule. Retinal and retinoic acid are structurally identical except for the addition of the oxygen atom. This change is necessary because transforming the aldehyde (CHO) group on the end of retinal to a carbolic acid (CHO2) group gives retinoic acid new biologically significant abilities. The downside is that this conversion is an irreversible process and vitamin A (in the form of retinoic acid) is no longer able to combine with the opsin protein.
Retinoic acid plays an important role in cell growth and differentiation because it's one of several molecules that are involved in gene expression. A Gene is defined as a segment of a chromosome on a DNA strand that contains the instructions for building a protein. When the gene is used to build a protein, it's said to be expressed. Retinoic acid binds to a retinoic acid receptor on the chromosome in a way that enables or prevents the gene from being expressed. Retinoic acid receptors are located at multiple positions on the chromosome, so the production of multiple proteins can be controlled. Some of the proteins that retinoic acid has influence over are involved in the growth and maintenance of epithelial cells. These are the cells that form the outer skin and the exposed surfaces of many organs, including the lungs and the urinary tract. This gives retinoic acid its ability to keep the skin healthy and enhance our immune systems. The body is able to fight off germs better when it has healthy surfaces.
|
Retinoic acid's ability to control gene expression is also extremely important for embrionic development. When a fertilized egg starts dividing into multiple cells, all of the cells are the same. To develop into an embryo, the cells need to differentiate into skin, nerve, muscle and many other types of cells. A stem cell does this by producing one set of proteins for a skin cell, another set for a blood cell, and so on. The proteins that are produced depend on which genes on the DNA molecule are turned on (expressed). Retinoic acid controls gene expression for many of the proteins that are involved in cell differentiation. If a mother doesn't consume enough vitamin A during pregnancy, the fetus can miscarry or become deformed.
The influence that retinoic acid has on a particular cell is also dependent on how much of it a cell receives from external sources. This enables the body to use retinoic acid as a chemical signal, controlling the cell's activity from outside the cell. This is how embrionic development works at the level of the entire organism. The position of cells relative to each other determines how much retinoic acid each cell receives, and therefore which proteins are expressed. Retinoic acid is also necessary for the production of sperm, the growth and maintenance of teeth and bones, and probably many other (as yet undiscovered) critical biological functions. So be sure to eat your liver and carrots.
After seeing all of the amazing things that retinal and retinoic acid can do, I'm sure you're wondering what retinol brings to the table. It is, after all, the third member of the vitamin A trilogy. Unfortunately, retinol doesn't seem to participate directly in any biologically transformative functions. It can't attach its hydroxyl group to any proteins that can use it as a co-enzyme. Its significant activity is limited to the transportation and storage of vitamin A.
Retinol is created from retinal by the retinal reductase enzyme, which attaches two additional hydrogen atoms to retinal's aldehyde (CHO) group. An NADPH molecule is converted into NADP+ in the process. This converts the aldehyde group into a new configuration with a hydroxyl (OH) group on its end, transforming it into a molecule called retinol. Retinol can be converted back into retinal by the alcohol dehydrogenase enzyme. When this happens, the two hydrogen atoms are removed when an NAD+ molecule is converted into NADH. Like the other two retinoids, retinol is unstable, and therefore unsuitable for storage or transportation. But its hydroxyl group gives it an ability that retinal and retinoic acid don't have. It can combine with a variety of acidic molecules into one of several molecules that are collectively called retinyl. The retinyl molecules are not only stable, their creation is reversible. This is the perfect combination of characteristics for the storage of a molecule that will be needed later.
Several different fatty acids (including palmitic, linoleic, oleic, and stearic acids) can be used when synthesizing retinyl, so retinyl refers to a group of different molecules that all store retinol in a stable, fat soluble form. They have names like retinyl palmitate and retinyl acetate, depending on the attached acid. Lecithin retinol acyltransferase combines (esterifies) retinol with a fatty acid to form a retinyl molecule, while retinyl ester hydrolase converts retinyl back into retinol. Some retinyl is stored in fat cells, but over 90% of it is stored in the liver, where they are created. Retinyl is the main form in which vitamin A is consumed when we eat meat (especially liver), because the animals we eat also store their vitamin A as retinyl. Our intestine can't aborb retinyl, though, so it's broken apart and absorbed as retinol, then recombined into retinyl in the liver.
|
|
While most retinol is stored in the liver as retinyl, most retinal and retinoic acid is needed in individual cells throughout the body. This is why retinol is also used as the form in which vitamin A is transported. When retinol is absorbed by the intestine, most of it is encapsulated into lipoprotein particles called Chylomicrons and transferred to the liver for storage as retinyl. When levels in the bloodstream are too low, the liver separates the retinol from retinyl and transfers it to a retinol-binding protein (RBP), which takes it to the cells that need it. RBP is necessary because retinol is a fat-soluble molecule and can't move through the watery bloodstream on its own. Even though retinol has a polarized hydroxyl (OH) tip, the larger non-polar (CH) bulk of the main molecule retains its non-polar influence, keeping it fat-soluble. When attached to RBP, the combined molecule is non-polar and therefore water-soluble.
When the combined molecule gets to its destination, RBP gives the retinol to a receptor on the cell membrane called STRA6, which transfers the retinol inside the cell. Cellular retinol-binding proteins (CRBPs) bind with the retinol for short term storage and intracellular transportation until it's converted to retinal or retinoic acid for use by the cell. Retinol is the perfect retinoid to store for later use because retinal can be converted into retinol, and then stored as retinyl. The retinyl can then be converted back into retinol as needed, which can then be converted into retinal or retinoic acid (via retinal) The conversion of retinal into retinoic acid is the only non-reversible reaction in the retinoid/retinyl group of reactions.
|
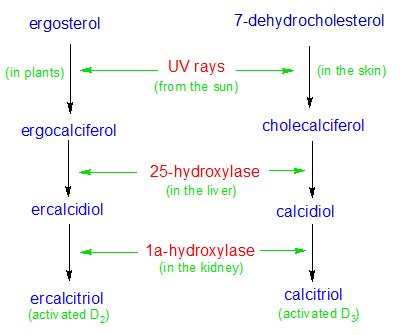
Vitamin D is a group of fat-soluble, steroid-like molecules that function like hormones in the body. The two most important molecules in the vitamin D family are ergocalciferol (Vitamin D2) and cholecalciferol (vitamin D3). These two forms of vitamin D are structurally identical (except for one bond), and perform similar functions in the human body. Vitamin D2 is made when ultraviolet light strikes the ergosterol molecule that's contained in a few types of plants (like phytoplankton, yeast and mushrooms). Most plants and animals are unable to produce Vitamin D2 because we don't have the ergosterol molecule. We make vitamin D3 instead, when ultraviolet light strikes the 7-dehydrocholesterol molecules in our skin.
Vitamin D snuck its way onto our list of vitamins under false pretenses. Vitamins are partly defined as essential nutrients, and vitamin D doesn't meet this standard. An essential nutrient is defined as a molecule that can't be produced by the human body. It must be acquired in our diet. This was thought to be the case with vitamin D, since its absence causes the disease called rickets. Since rickets is caused by a lack of vitamin D, it was assumed that the human body couldn't produce vitamin D on its own. The truth is that the human body can produce all of the vitamin D it needs when its skin is exposed to sunlight. People with rickets or osteomalacia were just not getting enough sunlight or had other issues that caused a deficiency of vitamin D (like liver or kidney damage).
Vitamin D performs multiple functions in the body, but is best known for its ability to regulate calcium and phosphorus levels in the blood. Calcium and phosphorus are used in many biological processes, and can't be transported throughout the body without vitamin D's assistance. Calcium is a major ingredient in the construction of bones and is used by nerve cells to transmit electrical signals. The transmission of nerve signals is required for everything from muscle contraction to the beating of the heart. When vitamin D isn't available to regulate calcium levels in the blood, the body is affected in many different ways.
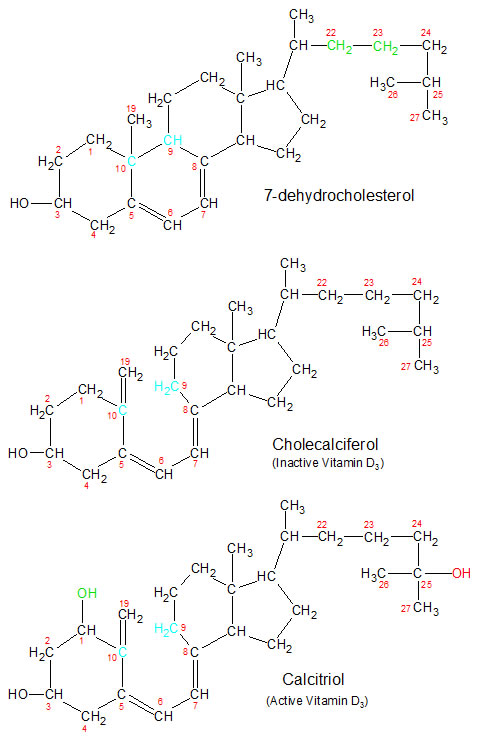
The series of biological reactions that result in the creation of vitamin D in humans begins in the skin on a sunny day. Ultraviolet rays from the sun are absorbed by a molecule of 7-dehydrocholesterol, which starts a series of reactions that convert it into a molecule of cholecalciferol (the inactive form of vitamin D3). A similar reaction creates inactive vitamin D2 (called ergocalciferol) out of ergosterol in some plants. The changes that take place in the molecular structures for vitamin D3 are depicted in the diagrams below. The molecular structures for ergosterol and its vitamin D2 derivatives are identical to the vitamin D3 molecules shown below, with one exception. Ergosterol has a double bond between carbons 22 and 23, while 7-dehydrocholesterol (shown below) has a single bond.
|
Whether you create vitamin D in your skin or absorb it from food, it's in an inactive state and needs to be transported to your liver (and then kidney) for activation. Both vitamin D2 and D3 are fat-soluble, and therefore must be transported throughout the body by the Vitamin D Binding Protein (DBP). This is true for all three (the inactive, intermediate and biologically active) forms of both vitamin D2 and D3.
In the case of vitamin D3, DBP transports the inactive form of vitamin D3 (derived from 7-dehydrocholesterol) to the liver where it's hydroxylated into calcidiol by the 25-hydroxylase enzyme (an OH group is attached to carbon number 25). DBP then transports it to the kidney, where it's hydroxylated yet again. This time the 1a-hydroxylase enzyme adds an additional OH group to vitamin D's number 1 carbon. It's now an activated form of vitamin D3 called calcitriol. DBP then transports calcitriol to any cells that can make use of it.
DBP transports vitamin D2 to the same locations, where it goes through the same processes as vitamin D3. The various forms of vitamin D2 have different names because vitamin D2 has a slightly different structure than vitamin D3, but their active components (the OH groups) are identical. The active form of vitamin D2 is called ercalcitriol.
Once the biologically active forms of D2 and D3 arrive at the cell where they are needed, DBP has completed its task. Vitamin D separates from DBP and enters the cell.
Vitamin D gets all of the credit for the body's ability to absorb and move calcium throughout the body, but it doesn't perform this function directly. It's involved in the creation of proteins that absorb and move the calcium ions.
Once inside a cell, the hydroxyl (OH) groups attached to carbons 3 and 25 of the vitamin D molecule form hydrogen bonds with locations on a vitamin D Receptor (VDR) molecule. This forms a two-molecule structure called a heterodimer, which binds to the surface of the cell's DNA. This activates the production of two proteins called TRPV6 and calbindin in a process called gene expression. It's these proteins that are directly involved in the absorption of calcium from the intestine into the cells lining the intestine wall. TRPV6 is a protein that inserts itself into the cell's membrane. It forms a channel in the cell wall that allows calcium to enter the cell. Calbindin is a protein that can bind to and transport calcium atoms. Calcium is then transported into the bloodstream.
How does the body regulate calcium and vitamin D levels in the blood? This is where the parathyroid glands enter the picture. The parathyroid glands monitor blood calcium levels. When these levels get too low, the glands start producing the parathyroid hormone (PTH). PTH activates 1a-hydroxylase, an enzyme in the kidney, which then converts calcidiol into calcitriol. Calcitriol activates the production of TRPV6 and calbindin in the cells, which enable the cells to absorb more calcium. When calcium levels get too high, the parathyroid glands stop producing PTH. Since fully activated vitamin D (calcitriol) has a very short half-life in the blood, this feedback loop is very responsive. Calcidiol has a much longer half-life than calcitriol, but it's not fully active and doesn't affect calcium blood levels. Calcitriol will only last in the bloodstream for a few days, but calcidiol can stick around for several weeks. Calcidiol's long half-life makes it the form of vitamin D measured when testing for vitamin D deficiencies.

Sardines (and other cold water fish) contain lots of vitamin D because they eat phytoplankton. 
We can produce vitamin D by exposing our skin to ultraviolet rays from the sun. |
Most of the foods we eat contain little or no vitamin D. If you aren't getting enough vitamin D from the sun, you can always fix up a nice plate of mushrooms, yeast or phytoplankton. If that doesn't sound tasty, you can eat something a little higher up the food chain. Fortunately for us, small cold-water fish (like sardines) eat lots of phytoplankton, and large cold-water fish (like tuna) eat lots of small cold-water fish. Since vitamin D is a fat soluble vitamin, it's stored in the liver and fatty tissues of these fish. This makes cold-water fish a great source of vitamin D.
If you're still not impressed with the dietary options for aquiring vitamin D, the USDA has come to your rescue. Since dietary sources of this vitamin are so limited, the USDA started adding vitamin D to milk years ago in an effort to eliminate rickets. More recently they've added it to additional foods like orange juice and eggs. Finally, if all else fails, you can always take a vitamin pill or go outside on a sunny day.
Vitamin D is a fat soluble vitamin, so it can accumulate in fatty tissues. While it's good to have a reserve supply for future use, vitamin D can be toxic if you get too much. Because vitamin D regulates levels of calcium in the blood, too much vitamin D can raise blood calcium levels too high. This can result in calcium being deposited in organs like the heart and kidneys where it will cause problems. When the kidneys get too much calcium, they create hard balls of calcium called kidney stones. Too much blood calcium can also cause problems with bones not forming in an optimal way.
Fifty micrograms per day is the recommended upper limit for daily vitamin D intake, with a recommended daily allowance (RDA) of five to fifteen micrograms. Skin cells regulate the production of vitamin D, so you won't reach toxic levels from too much sun. Food sources (even the fortified ones like milk) are low enough in vitamin D that you don't need to worry about getting toxic levels from a normal diet. Be careful with the vitamin pills, though. The parathyroid glands do their best to regulate calcium levels, but are no match for large doses of vitamin D from supplements consumed on a regular basis.
|
Vitamin E is created in the plastids of plants. Plastids are a family of organelles in plant cells that develop from a common proplastid into several functionally different organelles. Chloroplasts, chromoplasts, amyloplasts and some other organelles are all members of this group. Animal cells don't have plastids, so they can't create vitamin E.
There are two groups in the vitamin E family, each of which has four members called alpha, beta, gamma and delta. The groups are called the tocopherols and the tocotrienols, so you get individual molecules with compound names like alpha-tocopheral. The groups are defined by whether or not the string of carbon atoms attached to ring structure B (see the diagram) is fully saturated with hydrogen atoms. The tocopherol group's carbon chain is fully saturated, while the tocotrienol group has partially unsaturated carbons. Every forth carbon in the tocotrienol chain is connected to the next carbon by a double bond (shown in green) due to the missing hydrogen atoms.
While the two groups are different from each other due to the structures of their carbon chains, the molecules within each group differentiate themselves by how their A rings are structured. Carbons 5, 7 and 8 (in red) may or may not have CH3 groups attached to them. The alpha molecule has CH3 groups attached to all three carbons, while the gamma molecule doesn't have a CH3 group attached to carbon 5. This forces a re-positioning of the double bonds in the A ring of the gamma molecule relative to the alpha molecule.
All forms of vitamin E are absorbed from the intestine and into the lymphatic system where they are transported to the liver in chylomicrons. While the other forms of vitamin E have their functions to perform, alpha-tocopherol is the most biologically active form of vitamin E in the human body. This is because the alpha-tocopherol transfer protein (alpha-TTP) in the liver selectively transfers alpha-tocopherol into the bloodstream where it can reach the cells. This advantage (and the human body's inability to convert the other forms into alpha-tocopherol), gives alpha-tocopherol a near monopoly in the anti-oxidant role that vitamin E plays in cell membranes.
Oxidation is a natural biological process that usually (but not always) involves the oxygen atom. It's an essential reaction in any process where the partial or complete removal of an electron is necessary to change a molecule's biological activity. It's important to note that oxidation is only half of what's called a redox reaction. Any time one molecule loses an electron, another molecule gains an electron in an opposite process called reduction. Oxygen is more electro-negative than any other biologically useful element, so it's usually the atom that steals the electron. Oxygen oxidizes other atoms or molecules, and is reduced in the process.
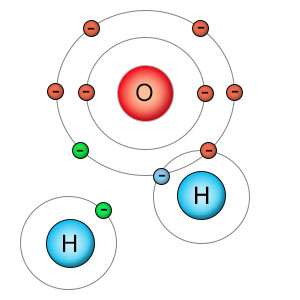
When a molecule of water is split apart into a hydrogen atom and a hydroxyl molecule, both parts have unpaired electrons (shown in green) which seek to pair up with electrons in other molecules. Oxygen is powerful enough to strip atoms from other molecules or combine with them in damaging ways. The hydrogen atom's nucleus is not powerful enough to remove electrons from other molecules or able to combine with them in a damaging way, so hydrogen isn't considered a free radical . |
Oxidation is defined on the energy page, but an example of how it works in a specific biological process wasn't given. Since vitamin E is used as an anti-oxidant in cell membranes, let's take a detailed look at oxidation in this scenario. We'll see how free radicals are created, how they are able to damage a lipid molecule, and how vitamin E repairs this damage or prevents it from happening. I'll use alpha-tocopherol and the hydroxyl free radical in this example because they are the most biologically active forms of both vitamin E and the oxygen-based free radicals in these types of reactions.
Free radicals are molecules that have unpaired electrons in their outer shells, and are very reactive as a result. This reactive ability is very useful under the right circumstances, but can cause damage when they react with the wrong molecules. Water (H2O) and oxygen (O2) molecules are not free radicals because they have no unpaired electrons, but they can both participate in reactions that can turn them into free radicals. O2 molecules accept an extra electron when they help turn sugar into energy during the forth stage of respiration. The extra electron is unpaired, so the O2 molecule becomes a free radical called superoxide. Water (H2O) can be split into hydrogen (H) and a hydroxyl molecule (HO) during hydrolysis and other chemical reactions. The hydroxyl molecule has an unpaired electron, making it a hydroxyl free radical. In many reactions the hydroxyl free radical is used in productive ways to create new molecules, but it can also react with molecules in destructive ways. Let's see how the hydroxyl radical reacts with an unsaturated fat in the cell wall.
The hydroxyl free radical is composed of one hydrogen atom bound to one oxygen atom. The hydrogen atom's single electron is bound to oxygen, so it has no unpaired electrons. The oxygen atom, however, has one unpaired electron. It's looking to form a bond with another atom. If it can find another hydrogen atom, it will bind with it and become a molecule of water. Lots of stable molecules in the cell have hydrogen atoms, and stealing from one of them will fill the void, but the molecule that loses its hydrogen atom will then become a free radical. This chain reaction will continue until a hydrogen atom is removed from an antioxidant.
Unsaturated fats are easy targets for free radicals. The double bonds between the unsaturated carbon atoms in the fatty acid chain reduce the strength of the single CH bonds in the adjacent carbons. The hydroxyl radical steals one of the weakly bound hydrogen atoms from the fatty acid chain in the cell membrane and becomes a water molecule. The fatty acid now has a carbon with an unpaired electron, and becomes a free radical itself. It can repair itself if it finds another hydrogen atom. But the carbon atom isn't as strong as an oxygen atom, so it's not as adept at stealing hydrogen atoms as the oxygen-containing molecules are. If a wandering (O2) molecule finds the unpaired electron on the fatty acid chain it will bind to it in a process referred to as lipid peroxidation. This will start a series of chemical reactions that will break apart the fatty acid chain into undesirable pieces, which cause the fat (or oil) to go rancid. Too many of these reactions will interfere with the cell membrane's ability to function properly, and can damage the cell.
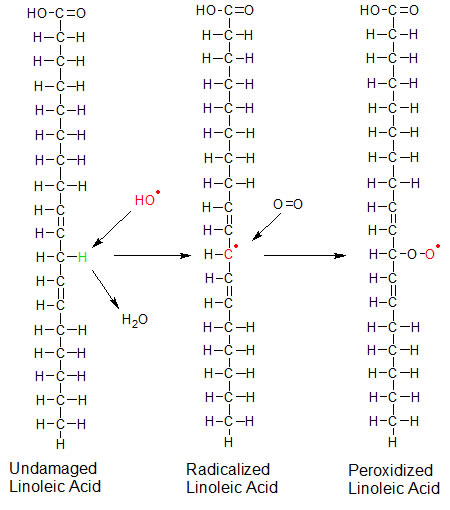
Linoleic acid is a polyunsaturated fatty acid that's used in cell membranes. In this example, a hydroxyl (OH) free radical removes the hydrogen and becomes water. The exposed carbon has an unpaired electron, making the fatty acid a free radical. An oxygen (O2) molecule then binds to the exposed carbon, becoming radicalized itself. The presence of oxygen on the fatty acid changes its structure. This damage can be prevented if vitamin E replaces the stolen hydrogen atom before any oxygen gets there. Radicalized atoms are shown in red. The stolen hydrogen is green. |
Lipid peroxidation can be prevented if the hydroxyl (HO) radical is intercepted by the vitamin E (alpha tocopherol) molecule before it finds the fatty acid. If this happens, the hydroxyl radical takes a hydrogen atom from vitamin E and becomes water. If the hydroxl radical has already damaged the fatty acid, vitamin E can repair the damage by replacing the fatty acid's stolen hydrogen atom with its own hydrogen atom before the lipid combines with oxygen. In either case the hydrogen atom from the OH group that's attached to carbon six of vitamin E's A ring is lost. This turns the vitamin E molecule (alpha-tocopherol) into a free radical.
If vitamin E becomes a free radical itself, how does this fix anything? Vitamin E is an antioxidant because it stops the oxidative chain-reaction that damages cells. While most molecules that have their hydrogen atoms stolen become free radicals that then steal a hydrogen atom from another molecule, vitamin E is a weak free radical. When it gives up its hydrogen atom it's too weak to steal one from most (but not all) other molecules. Most importantly, it's not strong enough to remove a hydrogen atom from a fatty acid.
The vitamin E free radical is weak because the ring structure of vitamin E redistributes some of the reactive power of its unpaired electron through a process called resonance stabilization. The oxygen with the unpaired electron is attached to carbon number six on ring A. Carbon six is attached to carbons five and seven. Both of these carbons have methyl (CH3) groups attached to them. All atoms in a molecule pull on the electrons of the other atoms in the molecule in varying degrees. This configuration of atoms interacts with the unpaired electron in a way that draws some of the reactive force of the unpaired electron into the ring of the molecule instead of outward towards other molecules. Alpha-tocopherol is more effective as an antioxidant than the other tocopherol forms of vitamin E because it has methyl groups on both of the carbons adjacent to carbon five. The other three tocopherols (beta, delta and gamma) have only one or no (delta) adjacent methyl groups.
Vitamin E's less reactive molecular form ends the chain reaction of hydrogen-stealing that damages lipids and other molecules (including proteins and DNA molecules), but it's not completely unreactive. The radicalized form of vitamin E can be reset through the donation of a hydrogen atom by another molecule that is willing to easily part with it. Vitamin C and glutathione are two such molecules. An alternative fate would be for vitamin E in its radical form to react with a second free radical. This would convert radical vitamin E into a non-reactive molecule like tocopherol quinone.

Swiss chard and other leafy greens are good sources of vitamin E. The cells in plant leaves are full of plastids like the chloroplasts and chromoplasts. Plastids are where vitamin E is created. |
The leaves of plants are a good source of vitamin E. Leaf cells contain lots of plastids, which is where vitamin E is produced. Vitamin E is also found in the cell walls of plants because it's a fat soluble vitamin and cell walls are composed mostly of fat molecules. Vitamin E molecules insert themselves into the cell walls of both plants and animals where they neutralize the free radicals that threaten the oxidation of fat molecules. This is how vitamin E gets its reputation as an antioxidant.
The best sources of vitamin E are found where the highest concentrations of fat molecules are located. This is why nuts and seeds are some of the best nutritional food sources for acquiring vitamin E. The grains are seeds to, and wheat germ is very high in vitamin E. Plants put lots of fat molecules into their seeds because seeds need lots of energy to germinate. Fat has more energy per gram than the carbohydrates, and seeds are small. Seeds use fat as a compact form of energy storage. Vitamin E is a fat-soluble vitamin, so it gravitates to where the fat is located. This is fortunate, since seeds often lie dormant for a long time before germinating and need protection from oxidation.

Sunflower seeds are high in vitamin E. |
Oxidation doesn't happen only inside of cells. Fat molecules that are exposed to oxygen in the atmosphere can become oxidized. When this happens to a bottle of unsaturated oil, the oil becomes rancid. This is why it's best to keep your oil sealed up where the air can't get to it. It's also why food producers prefer to use saturated fats in most of their food products. It gives them a longer shelf life.
Animals can't create vitamin E, but they can store it in their fatty tissues after eating plants. This means that vitamin E can also be acquired from fat-containing animal sources like liver and egg yolks. Since vitamin E is found in a wide variety of foods, deficiencies in this vitamin are rare. It should be noted that excessive heat can destroy vitamin E, so the best nutritional sources for it are from foods that you can prepare with minimal or no cooking.
Alpha-tocopherol is the only form of vitamin E that's included on nutrition labels since it's the most biologically active form of vitamin E. While the other forms of vitamin E can be consumed in the diet and absorbed by the intestine, alpha-tocopherol is the only form of vitamin E that's released from the liver into the bloodstream by the alpha-tocopherol transfer protein. The other members of the vitamin E family can't be converted into alpha-tocopherol, so they aren't considered a significant part of the human diet.
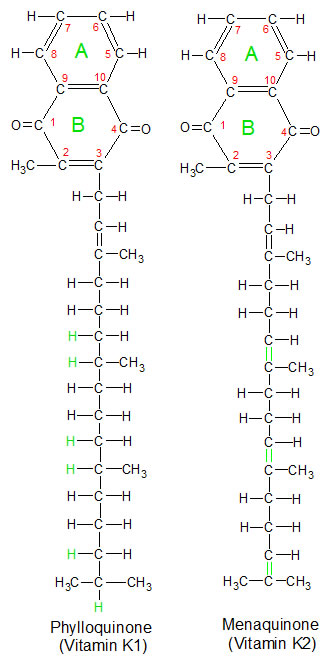
These two forms of vitamin K perform similar functions in the body because they have identical ring structures. They only differ in the number of hydrogen atoms attached to their carbon tails. |
Vitamin K is found in the diet in two forms called phylloquinone (vitamin K1) and menaquinone (vitamin K2). Phylloquinone is produced by plants, which use it as an electron acceptor in photosynthesis. The best food sources for phylloquinine are leafy green plants like romaine lettuce, because that's where photosynthesis takes place. Menaquinone is produced by bacteria in our intestines, so we get some vitamin K2 from them. Animals can convert excess phylloquinone into menaquinone and store it in their liver, so liver is the best dietary source for vitamin K2. Both forms can be used in our bodies because the biologically active portions of the molecules (carbon atoms 1 and 4 on ring structure B) are the same.  They differ only in the carbon chain attached to the ring structures. Since they are so similar in their biological activities, I'll refer to both of them as vitamin K for the rest of this article.
They differ only in the carbon chain attached to the ring structures. Since they are so similar in their biological activities, I'll refer to both of them as vitamin K for the rest of this article.
Vitamin K participates in a wide variety of biological processes. One process is the deposition of calcium in the bones. Another is the clotting of blood. Vitamin K takes part in these (and other) processes by providing energy that's needed for the completion of a reaction. This reaction converts an amino acid called glutamate (GLU) into another amino acid called gamma-carboxyglutamate (GLA). This reaction takes place in an area of a protein called the GLA domain. The GLA domain is a string of approximately forty five amino acids that contain nine to thirteen GLA's. The exact number depends on the protein being altered. There are several different proteins that contain GLA domains, and each type of protein performs a specific function. In all cases, vitamin K provides the same service. It acts as a molecular battery to provide energy for the conversion of GLU into GLA.
Vitamin K works with an enzyme called gamma-glutamyl carboxylase. These two molecules work together to carboxylate (add carbon dioxide to) glutamate (GLU), turning it into gamma-carboxyglutamate (GLA). GLA isn't one of the twenty amino acids that are added to proteins through gene expression, so it has to be created using this modification process. Once several of these conversions have been completed on a protein, that protein has a GLA domain. GLA domains are necessary because they bind to calcium ions. A GLA domain on an osteocalcin protein enables it to transport calcium to the bones, while a similar GLA domain on the blood protein prothrombin enables prothrombin to participate in the coagulation of blood.
The diagram below maps out a process called the vitamin K cycle. This same cycle is used on all GLA domain proteins, whether the final result is clotting of the blood, deposition of calcium in the bones or some other process. The ring portion of the vitamin K molecule is shown in the three forms that it takes during this cycle. To save space, I've replaced the carbon tail with the red letter R. You can see the tails for vitamin K1 and K2 in the diagram above this paragraph. The tails are not important in this process because the active areas on vitamin K are the H and O atoms attached to carbons 1 and 4 in the B ring structure. None of the protein molecules that contain a GLA domain are pictured in the diagram, but a single amino acid from a GLA domain is shown in both its GLU and GLA form. Let's go through the vitamin K cycle step by step.
|
We start the cycle with vitamin K quinone, which is the form in which we consume vitamin K. It's in an inactive state, like a battery that needs to be charged before it can be used. In step one we charge vitamin K by binding hydrogen atoms to the two oxygen atoms that are attached to carbons 1 and 4 in the B ring of vitamin K. This process is called reduction. There are two enzymes that can reduce vitamin K. The first is called vitamin K quinone reductase. This enzyme transfers one hydrogen atom and an additional electron from a molecule of NADH, converting it into NAD+. A nearby proton (a hydrogen atom with no electron) is combined with the extra electron to form the second hydrogen atom.
The second enzyme is called vitamin K epoxide reductase (VKOR), and doesn't need NADH. It provides the two hydrogen atoms from its own molecular structure. VKOR has two thiol (S-H) groups that combine into a single disulfide (S-S) group. The two hydrogen atoms released in this reaction bind to the oxygen atoms on vitamin K. The formation of the C-O-H bonds on vitamin K contain more energy than the C=O bonds, so the vitamin K molecule is now said to be charged. This new molecule is now called hydroquinone, or reduced vitamin K.
Step two is where vitamin K participates in the conversion of GLU to GLA. An enzyme called gamma-glutamyl carboxylase uses an oxygen (O2) molecule to remove the recently added hydrogen atoms from vitamin K. One of the oxygen atoms combines with the two hydrogen atoms to become a water molecule, which is discarded. The other oxygen atom binds to carbons 2 and 3, creating a third form of vitamin K called vitamin K 2,3 epoxide. This reaction releases the energy that was stored in step one. Gamma-glutamyl carboxylase uses this released energy in a coupled reaction to bind a molecule of carbon dioxide to a GLU amino acid in a reaction called carboxylation. This converts the GLU to GLA.
As we begin step three, we see that vitamin K 2,3 epoxide is the same as vitamin K quinone except for the extra oxygen that was bound to carbons 2 and 3 during step two. One of the two enzymes that can be used in step one (vitamin K quinone reductase) can't remove the extra oxygen, but the other enzyme (VKOR) can. VCOR uses the same two thiol groups as in step one, but this time the two hydrogens bind to the extra oxygen, separating it from carbons 2 and 3. This creates a molecule of water that's discarded. Vitamin K is now back in its original quinone form, and the cycle can start again. Recycling vitamin K in this manner greatly reduces our dietary needs for this vitamin.
While vitamin K is an essential participant in the processes that deposit calcium in our bones and cause our blood to clot, it does its work early in these complex processes, and far away from the areas where they have their final impact. Vitamin K does most of its work in the liver before the GLA domain molecules enter the bloodstream. To see how vitamin K fits into the bigger picture, let's take a quick look at the biological chain of events that lead to the clotting of blood after vitamin K helps to create a GLA domain on a prothrombin protein.
Prothrombin is a protein that's produced in the liver and circulates in the blood. It has an area in its amino acid string that contains ten glutamate (GLU) amino acids. Vitamin K provides the energy that converts the GLU into GLA, which can bind to calcium ions. When prothrombin binds to calcium ions it changes its shape in a way that helps it bind to lipid bilayers that are exposed when cells are damaged. This enables it to accumulate in the areas where clots need to form. It doesn't act alone, but combines with other molecules into a prothrombinase complex. While part of this complex, prothrombin is divided into fragments, one of which is a molecule called thrombin. Thrombin then removes the ends from soluble fibrinogen molecules (which also circulate in the blood). This creates insoluble fibrin, which combine to form a net that platelets get caught up in. This is how blood clots are formed. This process has many steps and involves several more molecules than I've mentioned, but this brief description gives you a larger overview of one of the biological pathways that vitamin K participates in.