
Garden and Plate
The Molecular Biology of Nutrition










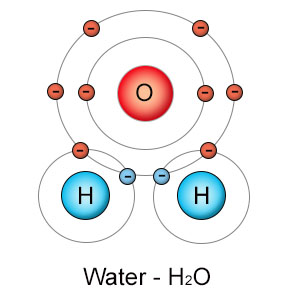
Energy is never created or destroyed, but it can change it's form and location. In molecular biology, energy is stored and released by the movement of electrons. Positively charged protons in the nucleus of an atom attract negatively charged electrons toward them, just as the sun attracts the earth. In this case, though, the attraction is caused by electromotive force (opposite charges), not gravity. When electrons are pulled closer to the nucleus of an atom, they release energy. When they are pulled away from the nucleus, they absorb energy. The tug of war that electrons are subjected to when atoms combine to form molecules and then break apart again is the means by which energy is stored and released in biological processes.

The burning of carbohydrates in a cell is the same chemical reaction that occurs when you burn wood in a fireplace. Wood, after all, is largely made of cellulose, which is a carbohydrate. In both cases, the carbohydrate is stable until a small amount of energy destablizes it, triggering the release of the energy within. In the case of wood in a fireplace, it's a match. In the cell, it's an enzyme. The only reason that cells don't burst into flame like wood is because the wood undergoes an uncontrolled chain reaction of energy release, while the enzyme controls the energy release in a cell.  Not only is the energy in a cell released in small steps, but if done correctly, most of it is absorbed by other molecules in a biologically productive way. In fact, the absorption of the released energy by other molecules is how many of the essential nutrients are created. This energy transfer isn't perfect. Some of it is dissipated as heat, which helps us maintain our 98 degree body temperature.
Not only is the energy in a cell released in small steps, but if done correctly, most of it is absorbed by other molecules in a biologically productive way. In fact, the absorption of the released energy by other molecules is how many of the essential nutrients are created. This energy transfer isn't perfect. Some of it is dissipated as heat, which helps us maintain our 98 degree body temperature.
To fully understand how cellular respiration (the controlled release of energy in a cell) goes about it's business without causing us to spontaneously combust when we eat a cookie, we need to examine a few things. We need to see how electronegativity allows molecules to maintain stable bonds, how enzymes are used to de-stablize those bonds, and how the energy-carrier molecules are used to store and release energy in small, biologically useful amounts.
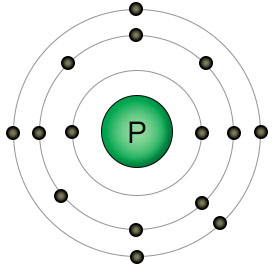
Phosphorus |
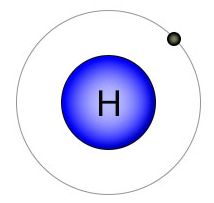
Hydrogen |
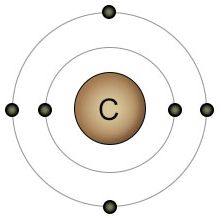
Carbon |
Oxygen |
Figure 1 - These four elements all play important roles in the transfer of energy between the energy carriers and many other molecules, including the carbohydrates. Energy is transferred from PO bonds in ATP to the HC bonds on glucose during photosynthesis. Energy is transferred from HC bonds on glucose to PO bonds on ATP during cellular respiration. |
Elements are defined by the number of protons in their nucleus, so no two elements have the same number of protons, or the same attractive force. All other things being equal, the element with the most protons has the most pull. But all other things aren't equal. Just as the force of gravity diminishes the farther away you get, so does the electromotive force. This is important in molecular biology because all chemical reactions take place in an atom's valence (outermost) shell. Elements that have lots of protons also attract lots of electrons, so their valence shell may be farther out. When you combine an element's pull with the distance of it's valence electron shell, you get it's effective attractive force. This attractive force is called electronegativity.
When you compare all twenty-one of the elements that are essential for plant and animal life, you'll find that oxygen has the highest electronegativity. It has eight protons, with two open slots in it's 2nd (valence) shell. There are biologically useful elements with more protons, but they also have more shells. Take phosphorus for example. It has fifteen protons, so you'd think it has a high electronegativity, but it also has three shells. Because phosphorus has three shells, it has a lower electronegativity than hydrogen. Hydrogen only has one proton, but it also only has one shell. Of the four elements shown in figure one, phosphorus and hydrogen have the lowest (almost identical) electronegativity, while oxygen has the highest. Carbon is somewhere in the middle.
It's also important to recognize that the attractive force (electronegativity) that a single atom has on it's electrons is reduced by the attractive force exerted by other atoms when covalent bonds are formed. Hydrogen's fragile grip on it's single electron is made even more tenuous when hydrogen and oxygen combine to form water. In this situation, separating hydrogen's nucleus (a single proton) from it's electron becomes even easier, because oxygen is already pulling hydrogen's electron away from hydrogen's nucleus. On the other hand, separating hydrogen's electron from oxygen's grip is much more difficult.
Pulling an electron away from an atom requires energy. Dropping an electron towards an atom releases energy. This is what makes chemical reactions possible. Since oxygen has more pull than any other biologically useful element, the removal of an electron from another atom is called oxidation (even when oxygen isn't involved). The addition of an electron to an atom is called reduction. In chemical reactions, oxidation and reduction occur simultaneously in what's called a reduction/oxidation (or redox) reaction. If oxygen pulls an electron from hydrogen, the oxygen atom is reduced (gains an electron) and the hydrogen atom is oxidized.
Most redox reactions aren't that clear cut. Instead of stealing hydrogen's electron entirely, oxygen and hydrogen often form a covalent bond. What happens then? Even though the electrons are shared, it's still a redox reaction if they are shared unequally. Since oxygen has a stronger electronegativity than hydrogen, it pulls the shared electron pair closer to it than to hydrogen. Hydrogen's electron falls down oxygen's electronegativity well, and energy is released. Because the sharing is unequal, oxygen is still reduced, and hydrogen is still oxidized. A particular element can be oxidized in some reactions and reduced in others. For example, when carbon binds to hydrogen, carbon is reduced. When carbon binds to oxygen, it's oxidized.
|
Now that we understand how differences in the electronegativity of the various elements are able to create redox reactions, it's time to ask the next question. How does this translate into productive biological activity? This is where enzymes come into the picture. Enzymes are proteins that disrupt the stable bonds in molecules so that the affected molecules (called substrates) can break apart and recombine in useful ways. Enzymes contain areas called "active sites" that attract and position specific molecules in such a way that the molecules participate in redox reactions.
Why can't molecules react with each other on their own? Some molecules can, but others can't. Most molecules are more stable than individual atoms because they've already participated in redox reactions when they formed their covalent bonds. That's why they're molecules. If a hydrogen atom meets up with an oxygen atom, they don't need an enzyme to help them combine in a redox reaction. They do it automatically because they both have open slots in their valence shells. But if a water molecule meets another molecule, it's less likely to react because the oxygen and two hydrogen atoms in water have already reacted with each other to form H2O, and are now stable. An enzyme can compromise that stability so that part or all of the hydrogen atom moves to another molecule, but that may take additional energy.
An enzyme not only assists in bringing the reactive molecules together, but it's active site contributes to the redox reaction by supplying an extra "pull" of it's own. Enzymes are used to couple the release of energy in one molecule to the absorbtion of energy in another. They bring the two molecules together, and also create an environment where it's easier for the energy transfer to take place. This is why enzymes are needed.
Enzymes are proteins, which can be produced or not depending on the biological needs of the organism. Since enzymes produce reactions that create specific biological molecules, the production of those molecules can be controlled by curtailing or encouraging the production of the related enzyme. This is how redox reactions are controlled in a way that supports productive biological activity.
Enzymes are not chemically changed at the end of the process, so they can be used over and over. Different molecules react under different conditions, so there are lots of different enzymes that each perform a specialized reaction.
If enzymes are expected to initiate redox reactions on demand, they need to have a ready supply of molecules (substrates) on hand. Most enzyme-assisted reactions involve both the splitting apart of one molecule and the stitching together of another. Due to differences in the electronegativity of the bonds involved, some of the reactions require an input of energy, while others generate a surplus. For example, photosynthesis uses energy to combine water and carbon dioxide into carbohydrate, while respiration produces energy by splitting carbohydrate apart into water and carbon dioxide. If extra energy is needed, it would be nice if a nearby molecule could donate some. If there's more energy than the reaction needs, it would also be nice if there was a molecule that could store it for later use. In both situations we get what we want. The molecules that perform these services are called the energy carriers.
The three most important energy carrier molecules are ATP, NADH and NADPH. These are the names given to them when they are "fully charged" and ready to donate electrons or phosphate groups. To be energy carriers, they must also be able to pick up electrons or phosphate groups to recharge. When they've donated their payloads and are "empty", they become ADP, NAD+ and NADP+. We'll take a quick look at all three before going into an in-depth example of how ATP is involved in the transfer of energy.

|
NADH and NADPH are identical molecules except for the addition of a phosphate group (PO3) to one of the nucleotides on NADPH. They each accept two additional electrons by adding a hydrogen atom and a free electron to their earlier forms (NAD+ and NADP+). Figure 5 shows the transition of NADP+ to NADPH. They differ from ATP in that they don't transfer phosphate groups.
The big difference between NADH and NADPH is in the reactions they participate in. NADPH donates it's electrons to chemical reactions that combine small molecules into larger ones. It's used in the Calvin Cycle to help create carbohydrates out of smaller molecules. NADH, on the other hand, donates it's electrons to chemical reactions that break molecules apart. NADH is used in glycolysis, where carbohydrates are broken down to provide energy for the creation of ATP out of ADP.
When plants convert the energy of the sun into ATP in photosynthesis, they do it by using the photons from the sun to excite electrons in magnesium ions. The excited electrons are then passed around until they are accepted by NADP+. That's how the NADPH gets created. The NADPH then donates it's electrons to a reaction that binds a phosphate group to a molecule of ADP. That's how plants create ATP.
Figure 4 - The two (red) PO bonds are split with help from an enzyme. The unattached phosphoryl group(s) can then be transferred by the enzyme to another molecule. |
ATP stands for adenosine triphosphate. It's called that because three phosphate groups are attached in series to an adenosine molecule, which in turn is composed of an adenine group and a ribose sugar. When ATP participates in redox reactions, it releases energy by transferring one or two of it's phosphate groups (PO3) to another molecule (with the help of an enzyme). ATP can donate up to two of it's three phosphate groups, becoming ADP or AMP in the process. AMP and ADP are then available to store excess energy from other energy-releasing processes (such as photosynthesis) by using that energy to re-attach the phosphate groups.
The phosphate groups are removed from ATP by breaking the P-O bonds that are shown in red in figure 4. These bonds don't dissolve spontaneously, because breaking bonds always requires an input of energy. In the example below, the energy released from the breakup of the PO bond in ATP is used to create a new PO bond when the severed phosphate group is attached to glucose.
When energy is released through the breakdown of a molecule like ATP, the energy release never takes place in isolation. The energy has to be transferred to something else. If another molecule can't use it for biologically significant purposes, it gets transferred to adjacent atoms or molecules as random motion (heat). In the example below, the Hexokinase enzyme not only initiates the breakdown of ATP to ADP and a phosphate group, it makes sure that there's a glucose molecule available to absorb the energy in a productive way. Let's take a detailed look at where the energy resides in an ATP molecule, and how it's used to transfer a phosphate group to glucose.

Figure 5 - The MG2+ ion in hexokinases' active site weakens the PO bond, which enables the hydrolysis (OH-) group to sever it. The OH group attaches to the phosphorus atom while the extra electron attaches to the oxygen atom that stays with the ADP molecule. |
Hexokinase is an enzyme that assists in the transfer of a phosphate group from ATP to glucose, which produces ADP and glucose-6-phosphate. This set of reactions is used in the first step of glycolysis (the breakdown of sugar).
The process begins when the positively charged MG2+ ion in hexokinase's active site attracts and binds to the negatively charged oxygen ions (O-) on either side of ATP's PO bond (one of the red bonds in figure 5). This puts stress on the PO bond, which weakens it. The amount of energy required to break the stable PO bond is now reduced to the point where a hydroxyl group can sever it.
At the same time, amino acids on hexokinase's active site form hydrogen bonds with the OH groups on the side of a glucose molecule, causing hexokinase to change it's shape. This in turn positions the glucose molecule close to the ATP molecule so that it can attach to ATP's phosphate group as soon as it's released.
With everything in place, a water molecule starts the process by separating from one of it's loosely-held hydrogen nuclei (a proton), to become a hydroxyl (OH-) ion. The negative charge (extra electron) on the hydroxyl ion weakens ATP's PO bond further, causing it to break apart in a process called hydrolysis (The oxygen atom in the PO bond accepts the extra electron, while the phosphorus atom binds to the oxygen atom on the remaining OH part of the hydroxyl ion).
Due to hexokinase's positioning of the ATP and glucose molecules, the OH group on the newly freed phosphoral group immediately comes into contact with the OH group that's attached to the carbon-6 atom on the glucose molecule. These two OH groups react in a process called condensation. Both of the hydrogen atoms and one of the two oxygen atoms combine to form a molecule of water (H2O), which is discarded. The remaining oxygen atom forms a bond that links the phosphoryl group to glucose, creating glucose-6-phosphate. This completes the process by which energy obtained from the breaking apart of one molecule is used to synthesize the creation of a new molecule.
Hexokinase is vital to this entire process not only because it weakens the PO bond in ATP, but because it couples two reactions (hydrolysis and condensation) together into one process by pre-positioning the ATP and glucose molecules. Why is this necessary? Because while hydrolysis produces a net gain of energy, condensation requires a net input of energy. Condensation can't take place without the energy made available by hydrolysis. Where does this energy come from and where does it go? Let's take a closer look.
If we compare the pre-process molecules of ATP and glucose with the post-process molecules of ADP and glucose-6-phosphate, we see that the total number and kinds of atoms are the same after the process completed as they were before it began. A water molecule was added during hydrolysis, but was then removed during condensation, so there was no change there. Hexokinase, like all enzymes, exited the process unchanged. The only change is in the positions of the atoms relative to each other, and the bonds that they've formed.

Figure 6 - The two OH groups only need one oxygen atom to combine Glucose and the phosphate group into glucose 6-phosphate. The rest forms a molecule of water which is discarded. This process uses energy that's provided by the hydrolysis reaction. Hexokinase (not shown) positions ATP and glucose so that the energy transfer can take place. |
The changes in bond formation require the electrons that make up the bonds to adjust to higher or lower energy states. This is how the energy was transferred, and why hydrolysis releases energy while condensation consumes it. When we look at the PO bond that binds the phosphoryl group to the rest of ATP, we see electrons forced into one configuration. When the hydroxyl group disrupts this configuration, energy is released in a number of ways.
The first way is called "Resonance Stabilization". When oxygen and phosphorus form a PO bond, they share electrons. These electrons are pulled into higher energy states by the opposite atom's nucleus. When the PO bond in ATP is broken, phosphorus establishes a new PO bond with the hydroxyl oxygen atom, so there's no energy change there. But the oxygen atom that's still attached to ADP isn't attached to the phosphorus atom anymore, so it's previously shared electron from the severed PO bond is now pulled in closer to oxygen's nucleus. When this happens, there's a release of energy.
The second way that energy is released during hydrolysis is called "Electrostatic Repulsion". Each of the three phosphate groups contain at least one negatively charged oxygen ion (O-). When the PO bonds are formed, the oxygen ions are forced close to each other. Since negative charges repel each other, it takes energy to force them together. It's like compressing a spring. When the PO bond is broken, the oxygen ions move apart, which releases the energy.
In a similar manner, since every phosphoryl group has a net negative charge, they repel each other after separation. So a phosphoryl group and a molecule of ADP has a lower energy state than a single molecule of ATP. The energy released by separation is increased by the effects of being separated in a solution of water. Both water and phosphoryl groups are polar molecules. The positive side of water attracts the negative side of a phosphoryl group, and the negative side of water attracts the positive side of a phosphoryl group. This further reduces the "spring-like" tension mentioned in the previous paragraph, by moving "like charges" farther apart. This is called the "Solvation Effect".
Some of the rearrangements that release energy during hydrolysis are reversed during condensation. When the phosphoryl group binds to glucose, a new PO bond is formed. It requires an input of energy for the same reasons that the breaking of ATP's PO bond released energy. Hexokinase positions ATP and glucose so that the energy released in hydrolysis is available for use in condensation. That's one example of how enzymes like hexokinase make the creation of nutritionally essential molecules possible.